49634
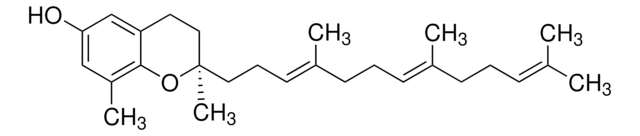
γ-Tocotrienol
analytical standard
Synonym(s):
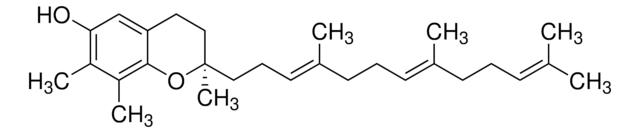
(R)-γ-Tocotrienol, [R-(E,E)]-3,4-Dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-2H-1-benzopyran-6-ol
Select a Size
About This Item
Recommended Products
biological source
Elaeis guineensis
Quality Level
grade
analytical standard
Assay
≥97.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
color
very light yellow to dark yellow
application(s)
pharmaceutical (small molecule)
vitamins, nutraceuticals, and natural products
format
neat
storage temp.
−20°C
SMILES string
C\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC[C@]1(C)CCc2cc(O)c(C)c(C)c2O1
InChI
1S/C28H42O2/c1-20(2)11-8-12-21(3)13-9-14-22(4)15-10-17-28(7)18-16-25-19-26(29)23(5)24(6)27(25)30-28/h11,13,15,19,29H,8-10,12,14,16-18H2,1-7H3/b21-13+,22-15+/t28-/m1/s1
InChI key
OTXNTMVVOOBZCV-WAZJVIJMSA-N
General description
Application
1 of 4
This Item | 900377 | T0452 | 68814 |
|---|---|---|---|
| grade analytical standard | grade - | grade - | grade analytical standard |
| format neat | format - | format - | format neat |
| biological source Elaeis guineensis | biological source - | biological source rice | biological source - |
| assay ≥97.0% (HPLC) | assay ≥96% (CP) | assay ≥75% (GC) | assay ≥97.0% (HPLC) |
| shelf life limited shelf life, expiry date on the label | shelf life - | shelf life - | shelf life limited shelf life, expiry date on the label |
| technique(s) HPLC: suitable | technique(s) mass spectrometry (MS): suitable | technique(s) - | technique(s) HPLC: suitable, gas chromatography (GC): suitable |
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.