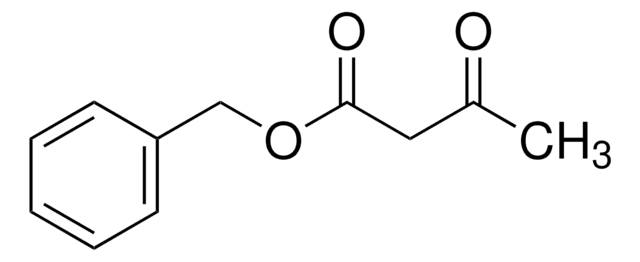
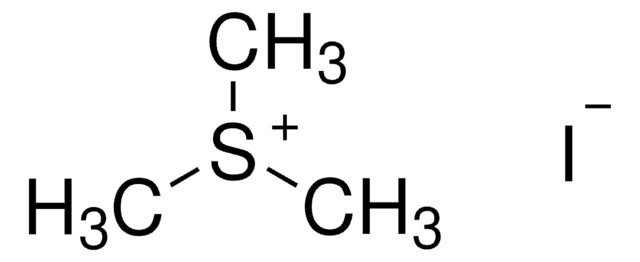
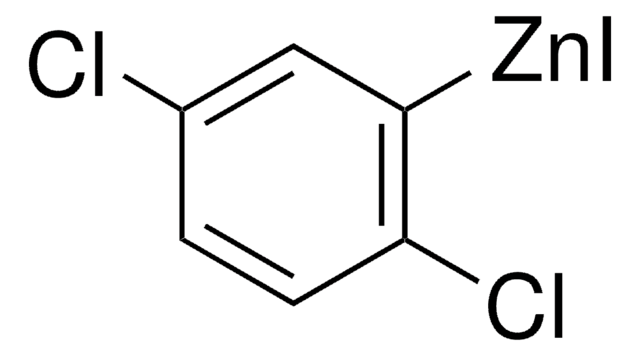
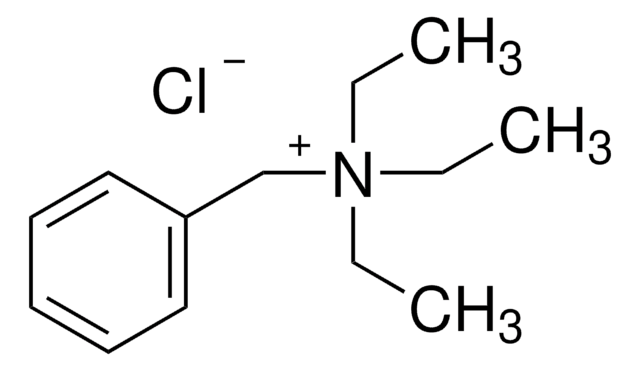
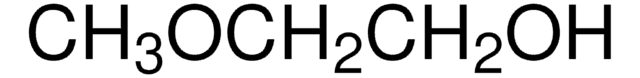D152927
Dimethyl carbonate
ReagentPlus®, 99%
Synonym(s):
Carbonic acid dimethyl ester
Select a Size
Select a Size
About This Item
Recommended Products
vapor density
3.1 (vs air)
Quality Level
vapor pressure
18 mmHg ( 21.1 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
dilution
(for general lab use)
refractive index
n20/D 1.368 (lit.)
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
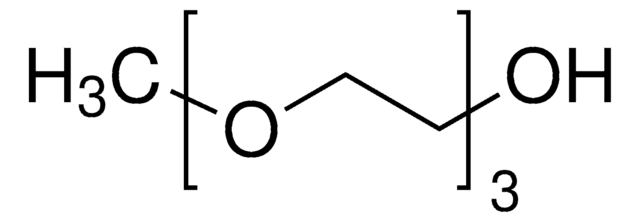
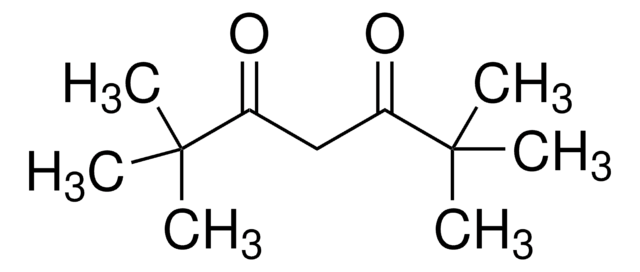
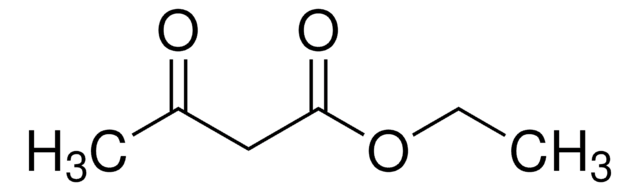
This Item | B88209 | 364789 | 441457 |
|---|---|---|---|
| assay 98% | assay ≥99% | assay 96% | assay 97% |
| Quality Level 100 | Quality Level 200 | Quality Level 200 | Quality Level 100 |
| bp 71-72 °C/11 mmHg (lit.) | bp 97-98 °C (lit.) | bp 65 °C/11 mmHg (lit.) | bp - |
| density 0.954 g/mL at 25 °C (lit.) | density 0.866 g/mL at 20 °C (lit.) | density 1.221 g/mL at 25 °C (lit.) | density - |
| refractive index n20/D 1.419 (lit.) | refractive index n20/D 1.386 (lit.) | refractive index n20/D 1.456 (lit.) | refractive index - |
| mp -38 °C (lit.) | mp - | mp 21 °C (lit.) | mp 157-163 °C (lit.) |
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is a Greener alternative to conventional solvents and chemicals. Click here for more information.
Application
Features and Benefits
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service