E1250
Elastase from porcine pancreas
Type I, ≥4.0 units/mg protein
Synonym(s):
Elastase from hog pancreas, Pancreatopeptidase E
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
Porcine pancreas
type
Type I
form
suspension
specific activity
≥4.0 units/mg protein
mol wt
25.9 kDa
contains
0.1% thymol
concentration
0.5-15.0 mg/mL in water
foreign activity
trypsin ≤50 BAEE units/mg protein
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Elastase from porcine pancreas has been used in a study to investigate the design, synthesis and evaluation of biomimetic affinity ligands for elastases. Elastase from porcine pancreas has also been used in a study to investigate the purification and partial characterization of the pancreatic proteolytic enzymes trypsin, chymotrypsin, and elastase.
Elastase from porcine pancreas has been used:
- to induce abdominal aortic aneurysm (AAA)
- to study the impact of indoleamine 2-3 dioxygenase 1 (IDO) in mice
- to digest aortas for aortic smooth muscle cells (SMC) isolation
- as a positive control of proteolytic digestion
The enzyme from Sigma has been used in the development of elastase-perfused animal model . This study determined if tobacco exposure could lower the threshold of aortic injury necessary for AAA (abdominal aortic aneurysm) development. It has also been used during the isolation of type II pneumocytes from human lungs.
Biochem/physiol Actions
Elastase hydrolyses elastin, the specific protein of elastic fibers, and digests hemoglobin, casein and fibrin.
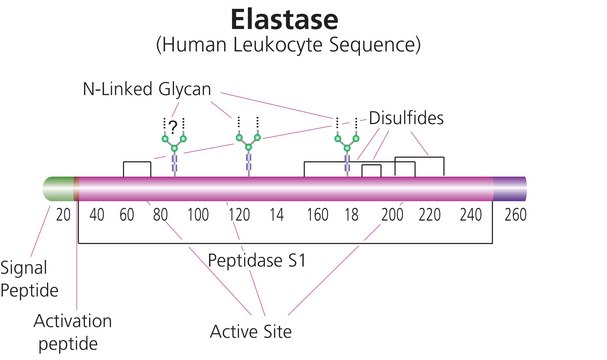
Elastase is a single polypeptide chain of 240 amino acid residues and contains four disulfide bridges. The molecular mass is approximately 25.9 kDa. The enzyme is synthesized as an inactive zymogen, proelastase, which is converted to the active form by limited proteolysis at the N-terminal by trypsin. It is a serine protease with broad specificity. It cleaves protein at the carboxyl side of small hydrophobic amino acids such as Ile, Gly, Ala, Ser, Val, and Leu. The enzyme also hydrolyzes amides and esters such as N-Benzoyl-L-alanine methyl ester. The pH optimum is found to be 8.0-8.5. It does not require any activator, but it is inhibited by diisopropyl fluorophosphate, phenylmethanesulfonyl fluoride, α2-macroglobulin, α1-antitrypsin, sulfonyl fluorides and p-dinitrophenyl diethylphosphate and high salt concentrations. It is extensively used in tissue and cell dissociation procedures. Elastase is effective in the isolation of Type II lung cells.
Packaging
Package size based on protein content
Unit Definition
One unit will hydrolyze 1.0 μmole of N-succinyl-L-Ala-Ala-Ala-p-nitroanilide per min, pH 8.0 at 25 °C.
Preparation Note
2× crystallized
Application
Product No.
Description
Pricing
inhibitor
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service