R2500
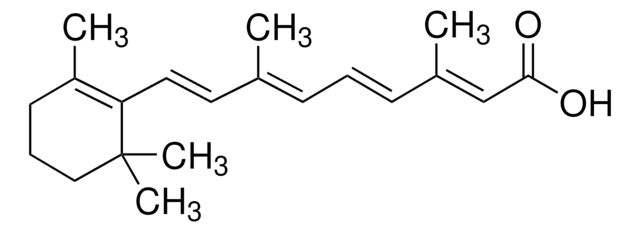
all trans-Retinal
powder, ≥98%
Synonym(s):
Vitamin A aldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C20H28O
CAS Number:
Molecular Weight:
284.44
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.79
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98%
form
powder
technique(s)
HPLC: suitable
color
yellow
mp
62-64 °C
shipped in
dry ice
storage temp.
−20°C
SMILES string
[H]C(=O)\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)CCCC1(C)C
InChI
1S/C20H28O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,15H,7,10,14H2,1-5H3/b9-6+,12-11+,16-8+,17-13+
InChI key
NCYCYZXNIZJOKI-OVSJKPMPSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
All trans-Retinal is one of the major derivatives of vitamin A group. A variety of food serves as a source of vitamin A. It is predominant in liver and among the brightly colored vegetables.
Application
All trans-Retinal has been used:
- in optogenetic experiments
- in electrophysiological experiment
- to study the effect of AKR1B10 (aldo-keto reductase (AKR) superfamily member) on the conversion of retinal to retinol in the airway epithelium
- in decidual transformation of human endometrial stromal cells
Biochem/physiol Actions
All-trans retinal is converted to retinoic acid in vivo by the action of retinal dehydrogenase. Retinoic acid is a ligand for both the retinoic acid receptor (RAR) and the retinoid X receptor (RXR) that act as transcription factors to regulate the growth and differentiation of normal and malignant cells. Retinal isomers are also chromophores that bind to opsins, a family of G-protein-linked transmembrane proteins, to form photosensitive receptors in visual and nonvisual systems. All-trans retinal is a potent photosensitizer.
Packaging
Sealed ampule.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service