242349
2-Chloroethyl methyl ether
98%
Synonym(s):
2-Methoxyethyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
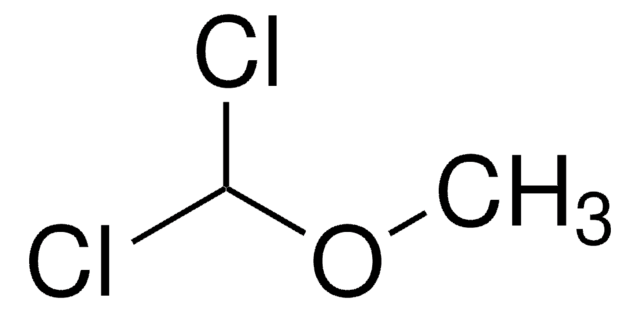
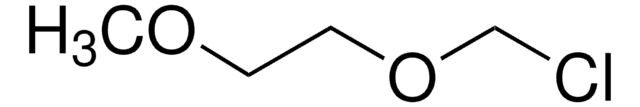
ClCH2CH2OCH3
CAS Number:
Molecular Weight:
94.54
Beilstein/REAXYS Number:
1731028
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.408 (lit.)
bp
89-90 °C (lit.)
density
1.035 g/mL at 25 °C (lit.)
functional group
chloro
ether
SMILES string
COCCCl
InChI
1S/C3H7ClO/c1-5-3-2-4/h2-3H2,1H3
InChI key
XTIGGAHUZJWQMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Chloroethyl methyl ether (2-Methoxyethyl chloride) was used in the synthesis of acyclic nucleosides of thieno[2,3-d]pyrimidine derivatives.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Irrit. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
46.4 °F - closed cup
flash_point_c
8 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2-[2-(2-Chloroethoxy)ethoxy]ethanol 96%](/deepweb/assets/sigmaaldrich/product/structures/902/295/ff6d7bb1-a7e0-4582-86e1-819084626e67/640/ff6d7bb1-a7e0-4582-86e1-819084626e67.png)