381438
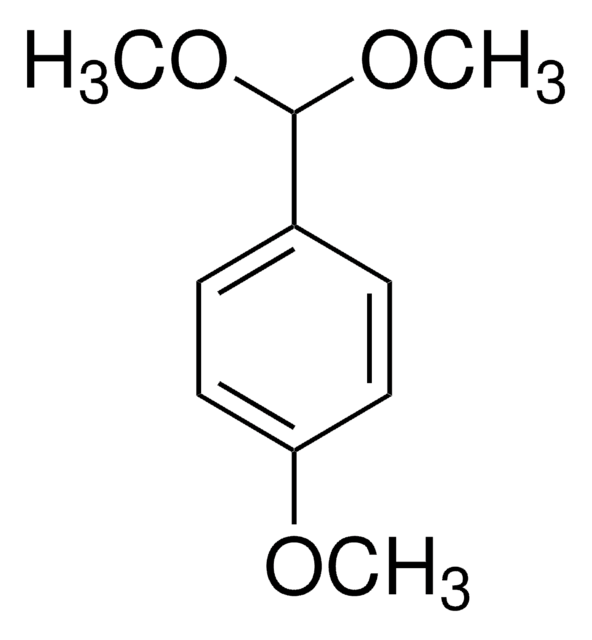
Benzaldehyde dimethyl acetal
95%
Synonym(s):
α,α-Dimethoxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH(OCH3)2
CAS Number:
Molecular Weight:
152.19
Beilstein/REAXYS Number:
2044501
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
refractive index
n20/D 1.493 (lit.)
bp
87-89 °C/18 mmHg (lit.)
density
1.014 g/mL at 25 °C (lit.)
functional group
acetal
ether
phenyl
SMILES string
COC(OC)c1ccccc1
InChI
1S/C9H12O2/c1-10-9(11-2)8-6-4-3-5-7-8/h3-7,9H,1-2H3
InChI key
HEVMDQBCAHEHDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
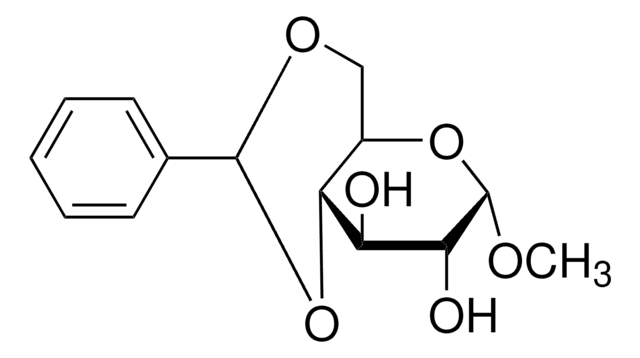
Benzaldehyde dimethyl acetal is an organic building block. Pyridinium tosylate-catalyzed acetal exchange reaction between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-α-D-glucofuranose is reported to afford 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranose. The kinetics of the hydrolysis of benzaldehyde dimethyl acetal over amberlite IR-120 has been studied using a circulated batch reactor in dioxane. One-pot tandem conversion of benzaldehydedimethylacetal to trans-1-nitro-2-phenylethylene has been reported.
Application
Benzaldehyde dimethyl acetal is suitable for use in the synthesis of 4,6-dihydroxy sugar, required for the total synthesis of Porphyromonas gingivalis 381 derived lipid A. It may be used in the preparation of 1-O-methyl-2,3-di-O-galloyl-β-D-glucose.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
156.2 °F - closed cup
flash_point_c
69 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service