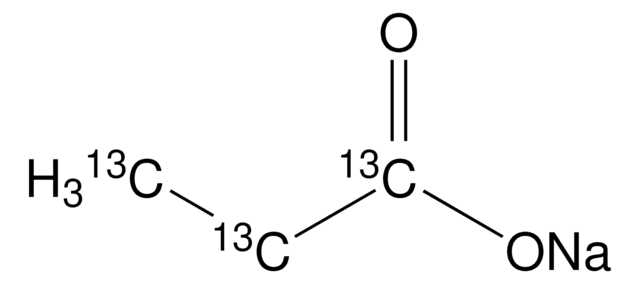
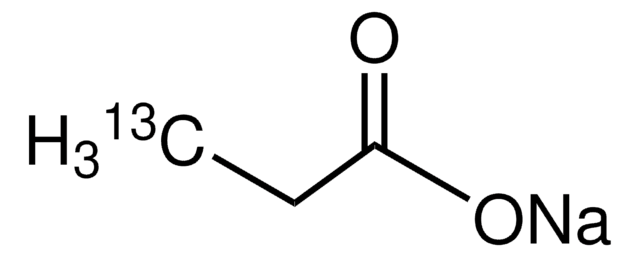
A96150
Azelaic acid
technical grade, 80%
Synonym(s):
1,7-Heptanedicarboxylic acid, Anchoic acid, Nonanedioic acid
About This Item
grade
technical grade
vapor density
6.5 (vs air)
vapor pressure
<1 mmHg ( 20 °C)
assay
80%
form
powder
bp
286 °C/100 mmHg (lit.)
mp
109-111 °C (lit.)
SMILES string
OC(=O)CCCCCCCC(O)=O
InChI
1S/C9H16O4/c10-8(11)6-4-2-1-3-5-7-9(12)13/h1-7H2,(H,10,11)(H,12,13)
InChI key
BDJRBEYXGGNYIS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 490636 | 490679 | 279455 |
|---|---|---|---|
| mass shift M+3 | mass shift M+3 | mass shift M+1 | mass shift M+1 |
| isotopic purity 99 atom % 13C | isotopic purity 99 atom % 13C | isotopic purity 99 atom % 13C | isotopic purity 99 atom % 13C |
| form solid | form solid | form solid | form solid |
| mp 285-286 °C (lit.) | mp 285-286 °C (lit.) | mp 285-286 °C (lit.) | mp 285-286 °C (lit.) |
| suitability endotoxin tested | suitability - | suitability - | suitability - |
General description
Application
- Synthesis of pH-responsive metallo-hydrogels as potential dye-adsorbing agents, and vitamin B12 carriers.[2]
- Synthesis of fluorescent uranyl (UO22+) coordination polymers.[3]
- Synthesis of various metal-organic frameworks (MOFs) and supramolecular macrocyclic adducts.[4][5]
- Conversion of hydrophobic up-converting rare-earth nanophosphors (UCNPs) into water-soluble, carboxylic acid-functionalized analogs to facilitate conjugation with biomolecules such as streptavidin.[6]
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
410.0 °F - closed cup
flash_point_c
210 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service