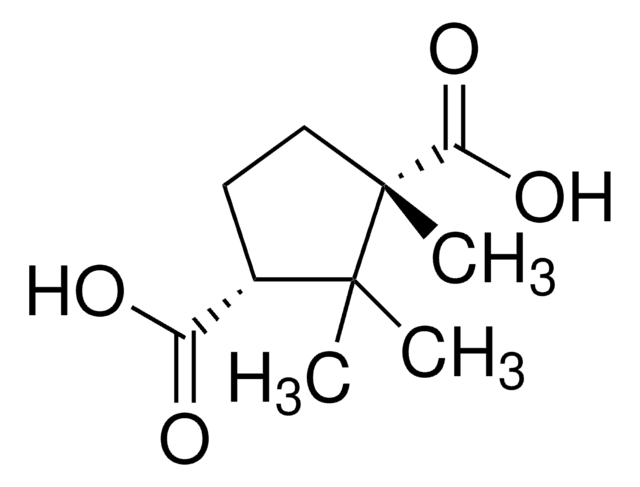
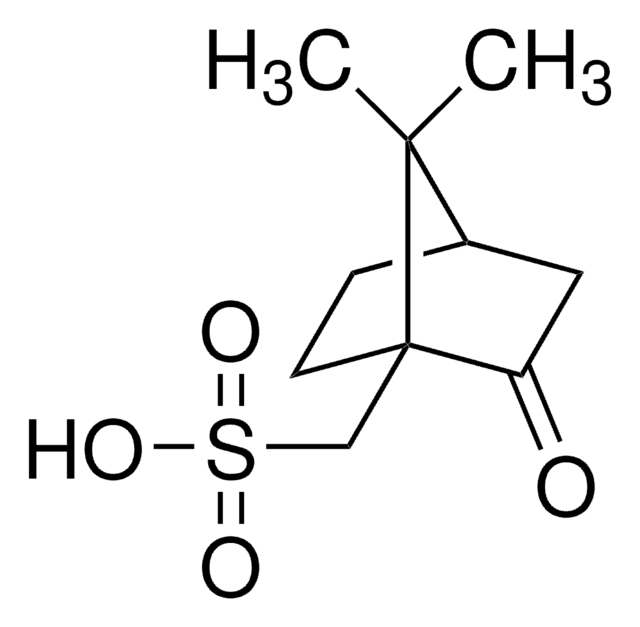
C409
(1R,3S)-(+)-Camphoric acid
99%
Synonym(s):
(+)-Camphoric acid, (1R,3S)-1,2,2-Trimethyl-1,3-cyclopentanedicarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H16O4
CAS Number:
Molecular Weight:
200.23
Beilstein/REAXYS Number:
2050204
EC Number:
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
powder
optical activity
[α]20/D 46°, c = 1 in ethanol
mp
183-186 °C (lit.)
SMILES string
CC1(C)[C@H](CC[C@@]1(C)C(O)=O)C(O)=O
InChI
1S/C10H16O4/c1-9(2)6(7(11)12)4-5-10(9,3)8(13)14/h6H,4-5H2,1-3H3,(H,11,12)(H,13,14)/t6-,10+/m1/s1
InChI key
LSPHULWDVZXLIL-LDWIPMOCSA-N
Related Categories
General description
Camphoric acid is a diacid, generally prepared by the oxidation of terpene (+)-camphor. It can be used as a chirality inducing agent in some organic reactions.
Application
(1R,3S)-(+)-Camphoric acid may be used in the preparation (1R,2S,3R,5S)-2,3-dibenzyl-1,8,8-trimethyl-3-thianiumbicyclo[3.2.1]octane perchlorate. It reacts with uranyl nitrate in pyridine(py) or py/methanol(MeOH) to form novel uranyl-organic assemblages.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service