W439400
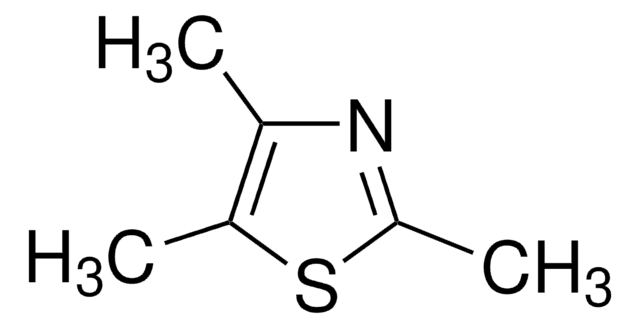
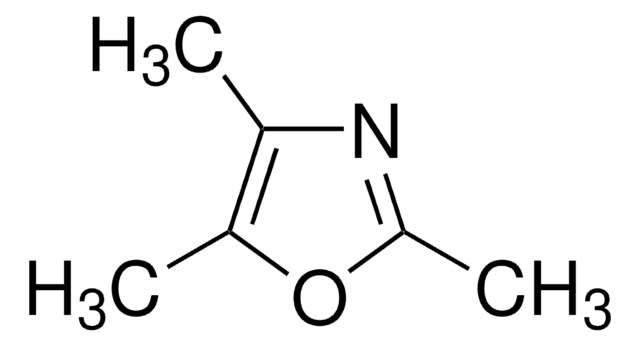
2,4,5-Trimethyloxazole
99%, FG
Synonym(s):
Trimethyloxazole
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
reg. compliance
EU Regulation 1334/2008 & 872/2012
assay
99%
refractive index
n20/D 1.442 (lit.)
bp
133-134 °C/760 mmHg (lit.)
density
0.957 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | W371602 | W330604 | W511609 |
|---|---|---|---|
| grade FG | grade FG, Halal, Kosher | grade FG, Fragrance grade, Halal, Kosher | grade - |
| organoleptic green; nutty; sweet | organoleptic green; vegetable; nutty | organoleptic hazelnut; musty; nutty; peanut | organoleptic coffee; nutty; roasted |
| biological source synthetic | biological source synthetic | biological source synthetic | biological source synthetic |
| food allergen no known allergens | food allergen no known allergens | food allergen no known allergens | food allergen no known allergens |
| documentation see Safety & Documentation for available documents | documentation see Safety & Documentation for available documents | documentation see Safety & Documentation for available documents | documentation see Safety & Documentation for available documents |
| assay 99% | assay ≥98% | assay ≥97% | assay ≥97% |
General description
Application
Biochem/physiol Actions
Taste at 1ppm: Sweet roasted cocoa, coffee, chocolate, slightly seared savory beef, proteinous with green vegetative nuances.[3]
Other Notes
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![5H-5-Methyl-6,7-dihydrocyclopenta[b]pyrazine ≥97%, FG](/deepweb/assets/sigmaaldrich/product/structures/977/118/0f103486-e428-4abd-a429-3075804ae8e8/640/0f103486-e428-4abd-a429-3075804ae8e8.png)