8.52220
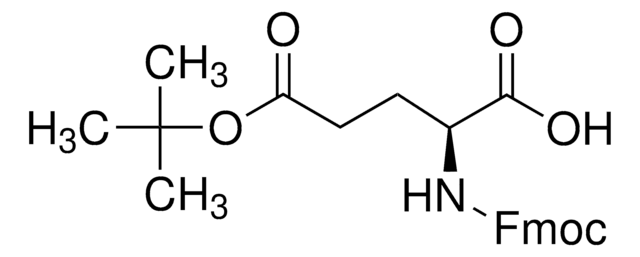
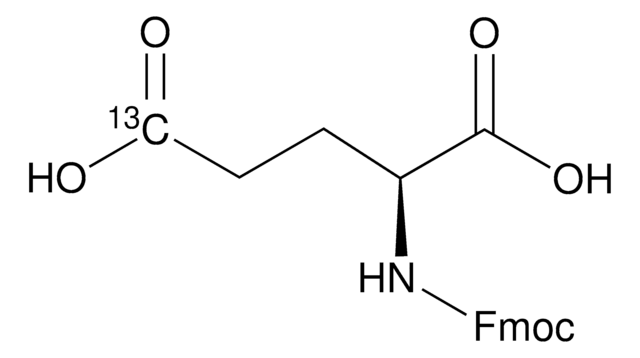
Fmoc-Asp-OBzl
Novabiochem®
Synonym(s):
Fmoc-Asp-OBzl, N-α-Fmoc-L-aspartic acid α-benzyl ester
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
assay
≥90.0% (acidimetric)
≥98% (TLC)
≥98.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
carboxylic acid
storage temp.
2-30°C
SMILES string
N([C@@H](CC(=O)O)C(=O)OCc4ccccc4)C(=O)OCC1c2c(cccc2)c3c1cccc3
1 of 4
This Item | 517127 | 8.03525 | 13259 |
|---|---|---|---|
| Quality Level 200 | Quality Level 100 | Quality Level 200 | Quality Level 100 |
| assay 99% | assay ≥99% | assay ≥99.0% (GC) | assay ≥99.0% (GC) |
| form liquid | form liquid | form liquid | form - |
| bp 90 °C (lit.) | bp 90 °C (lit.) | bp 90 °C/1016 hPa | bp 90 °C (lit.) |
| density 1.069 g/mL at 25 °C (lit.) | density 1.069 g/mL at 25 °C (lit.) | density 1.07 g/cm3 at 20 °C | density 1.069 g/mL at 25 °C (lit.) |
| mp 2-4 °C (lit.) | mp 2-4 °C (lit.) | mp 0.5-4.7 °C | mp 2-4 °C (lit.) |
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Need A Sample COA?
This is a sample Certificate of Analysis (COA) and may not represent a recently manufactured lot of this specific product.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Related Content
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service