Recommended Products
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
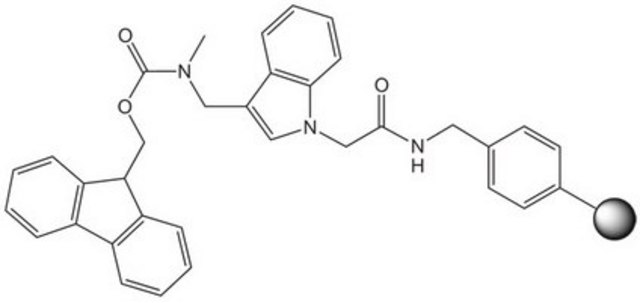
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
aldehyde
storage temp.
2-8°C
1 of 4
This Item | 8.55116 | 8.55018 | 8.55008 |
|---|---|---|---|
| reaction suitability reaction type: Fmoc solid-phase peptide synthesis | reaction suitability reaction type: Fmoc solid-phase peptide synthesis | reaction suitability reaction type: Fmoc solid-phase peptide synthesis | reaction suitability reaction type: Fmoc solid-phase peptide synthesis |
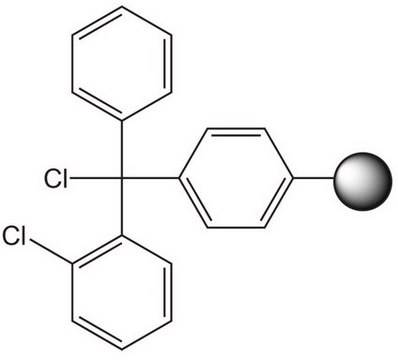
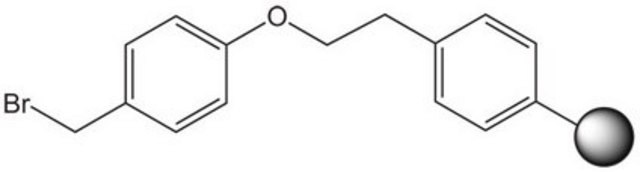
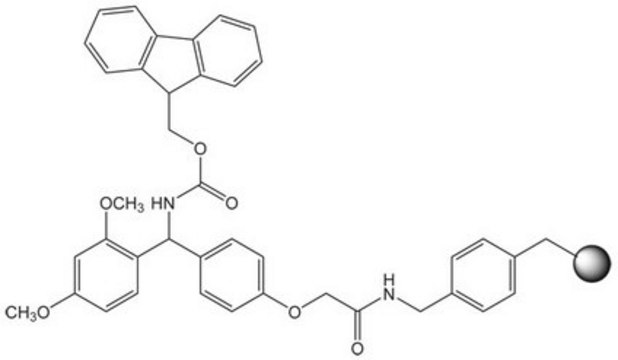
| functional group aldehyde | functional group - | functional group alcohol | functional group amine |
| product line Novabiochem® | product line Novabiochem® | product line Novabiochem® | product line Novabiochem® |
| Quality Level 200 | Quality Level 300 | Quality Level 200 | Quality Level 400 |
| application(s) peptide synthesis | application(s) peptide synthesis | application(s) peptide synthesis | application(s) peptide synthesis |
| storage temp. 2-8°C | storage temp. 2-8°C | storage temp. 2-30°C | storage temp. 2-8°C |
General description
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] K. G. Estep, et al. (1998) J. Org. Chem., 63, 5300.
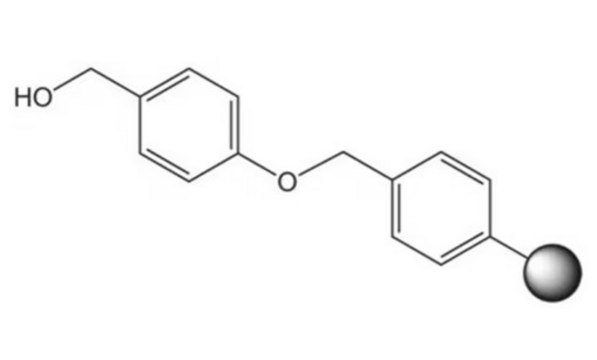
Linkage
Analysis Note
Appearance of substance (visual): beads
Loading (Photometric determination of the Fmoc-chromophore liberated upon treatment with DBU/DMF): 0.80 - 1.10 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 100 - 200 mesh
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Need A Sample COA?
This is a sample Certificate of Analysis (COA) and may not represent a recently manufactured lot of this specific product.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service