NGLYFL-RO
Roche
N-Glycosidase F
recombinant form of the gene from Flavobacterium meningosepticum
Synonym(s):
N-Glycosidase F, PNGase F, Peptide-N-glycosidase F, Peptide-N4-(acetyl-β-glucosaminyl)-asparagine amidase
About This Item
Recommended Products
biological source
bacterial (Flavobacterium meningosepticum)
Quality Level
recombinant
expressed in E. coli
conjugate
(N-linked)
assay
≥90% (SDS-PAGE)
form
lyophilized
specific activity
>25000 units/mg protein
mol wt
35.5 kDa
purified by
electrophoresis
packaging
pkg of 100 U (11365185001)
pkg of 250 U (11365193001)
manufacturer/tradename
Roche
storage condition
(Keep container tightly closed in a dry and well-ventilated place.)
technique(s)
activity assay: suitable
color
colorless
optimum pH
7.0-8.0
solubility
water: soluble
suitability
suitable for enzyme test
foreign activity
Endoglycosidase F, none detected
a-Fucosidase, present
b-Galactosidase, present
b-N-Acetylhexosaminidase, present
dA(17h ≤100 units, present
storage temp.
2-8°C
General description
N-glycosidase F, also known as PNGase F, is an asparagine amidase enzyme derived from Flavobacterium meningosepticum. It is widely used as a valuable tool in protein research to investigate and analyze N-glycosylation.
Specificity
Application
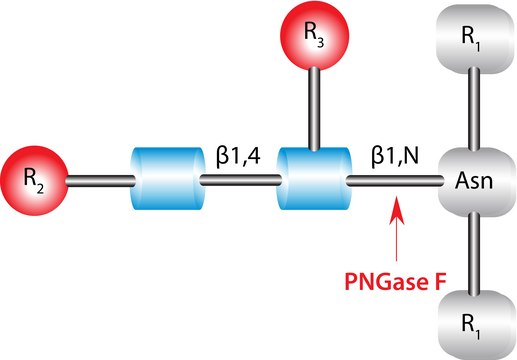
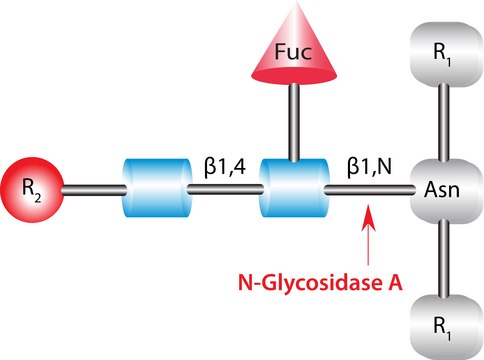
Use N-glycosidase F to cleave all types of asparagine-bound N-glycans, provided that the amino group as well as the carboxyl group are present in a peptide linkage, and that the oligosaccharide has the minimum length of the chitobiose core unit. The reaction products are ammonia, aspartic acid (in the peptide chain), and the complete oligosaccharide.
Note: N-Glycosidase F, recombinant is also available as a solution.
Unit Definition
Physical form
Preparation Note
The reconstituted solution is stable at 2 to 8 °C for at least four weeks.
Reconstitution
Note: N-Glycosidase F, recombinant is also available as solution with 50% glycerol.
Other Notes
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 2
flash_point_f
does not flash
flash_point_c
does not flash
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service