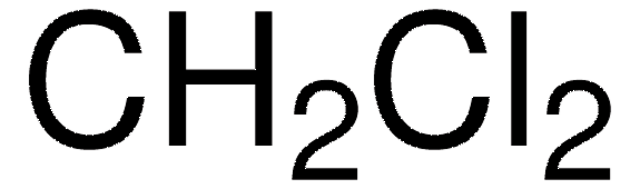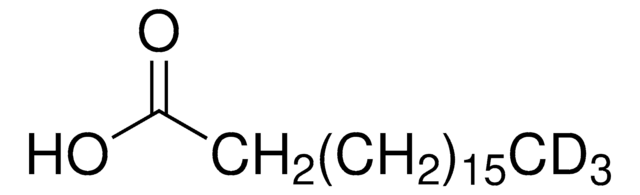01829
Extractables and Leachables Screening Standard for GC
certified reference material, TraceCERT®, 14 components in tert-Butyl methyl ether, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
concentration
50 mg/L each component (nominal concentration)
application(s)
pharmaceutical
format
multi-component solution
storage temp.
−20°C
1 of 4
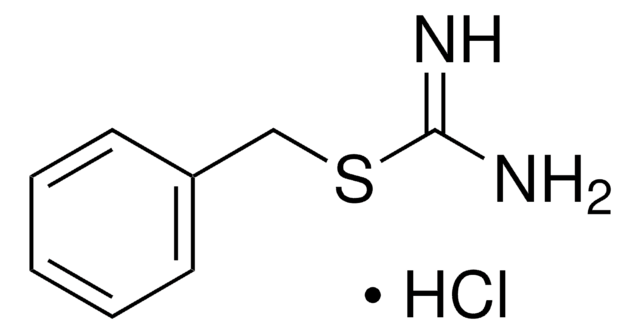
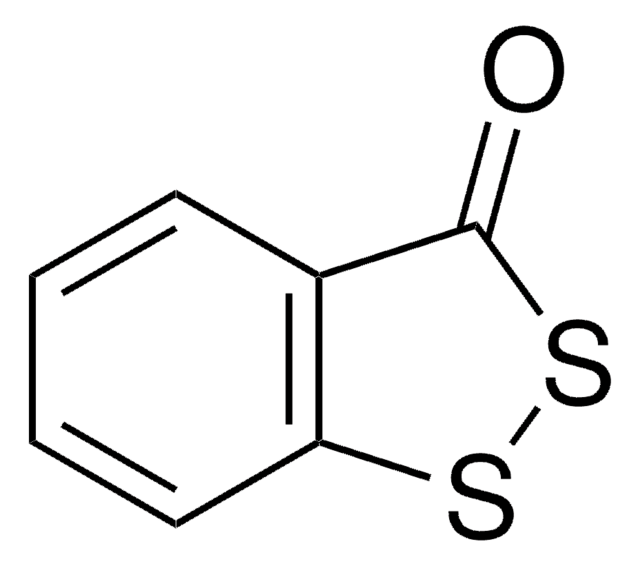
This Item | 251038 | 375462 | 284297 |
|---|---|---|---|
| assay ≥98% | assay 98% | assay 97% | assay 98% |
| solubility 95% ethanol: soluble 50 mg/mL, clear to very slightly hazy, colorless to yellow, diethyl ether: soluble, propylene glycol: soluble, water: insoluble, benzene: soluble, toluene: soluble, isopropanol: soluble | solubility methanol: soluble 1 g/10 mL, clear, colorless to very faintly yellow | solubility toluene: soluble 2.5%, clear, yellow | solubility methanol: soluble 50 mg/mL, clear to slightly hazy, colorless to yellow |
| mp 158-160 °C (lit.) | mp 177-179 °C (lit.) | mp 74-77 °C (lit.) | mp 228-231 °C (lit.) |
| Quality Level 100 | Quality Level 200 | Quality Level 100 | Quality Level 100 |
General description
Certified content incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Check out our complete range of extractables and leachables certified reference mixes
Application
Preparation Note
Legal Information
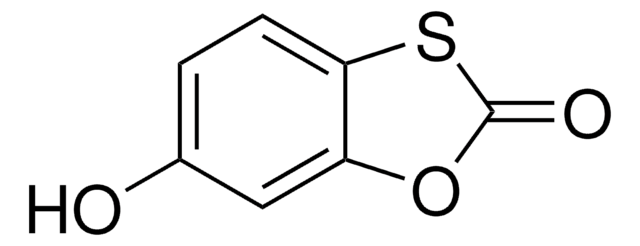
Analyte
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2 - Skin Irrit. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
-18.4 °F - closed cup
flash_point_c
-28 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service