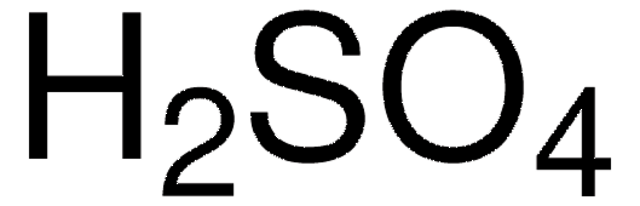68279
Sulfuric acid concentrate
0.1 M H2SO4 in water (0.2N), eluent concentrate for IC
Synonym(s):
Sulfuric acid solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2SO4
CAS Number:
Molecular Weight:
98.08
Beilstein/REAXYS Number:
2037554
MDL number:
UNSPSC Code:
12161700
PubChem Substance ID:
NACRES:
NB.21
Recommended Products
Quality Level
form
liquid
concentration
0.1 M H2SO4 in water (0.2N)
technique(s)
ion chromatography: suitable
SMILES string
OS(O)(=O)=O
InChI
1S/H2O4S/c1-5(2,3)4/h(H2,1,2,3,4)
InChI key
QAOWNCQODCNURD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Sulfuric acid (hydrogen sulfate, sulphine acid,battery acid) is a clear, colorless, oily liquid and is very corrosive in nature towards both metal and tissues. It is soluble in water.
This eluent concentrate for ion chromatography is determined by potentiometric titration. Content and expiry date can be found on the certificate.
Application
Metrohm IC application note AN-S-344:Anions in "Electronic grade" nitric acid on a high-capacity column.
Metrohm IC application note AN-S-341:4-Hydroxybutyratein addition to standard anions and organic acids.
Metrohm IC application note AN-S-336:Anion traces on the Metrosep A Supp 16 - 250/2.0 after Inline Preconcentration and Matrix Elimination
Metrohm IC application note AN-S-341:4-Hydroxybutyratein addition to standard anions and organic acids.
Metrohm IC application note AN-S-336:Anion traces on the Metrosep A Supp 16 - 250/2.0 after Inline Preconcentration and Matrix Elimination
Biochem/physiol Actions
It finds application in the manufacture of explosives, fertilizers, glue and other acids. It is also used in the pickling of metal, purification of petroleum, and in lead-acid batteries. It is widely used as dehydrating agent, catalyst, and as an active reactant in chemical industry. It is also suitable to control pH, ranging from saline solutions to strong fuming acids.
Sulfuric acid is prepared by dry distillation of minerals like copper sulfate pentahydrate (blue vitriol), and iron sulfate heptahydrate (green vitriol). On application of heat these compounds decompose to form iron and copper oxides thereby eliminating water and sulphur trioxide, which then combine to give, dilute solution of sulphuric acid.
Linkage
Visit the IC Portal to learn more
Preparation Note
Prepared with sulfuric acid and high purity water (18.2 MΩ, 0.2 μm filtered)
related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service