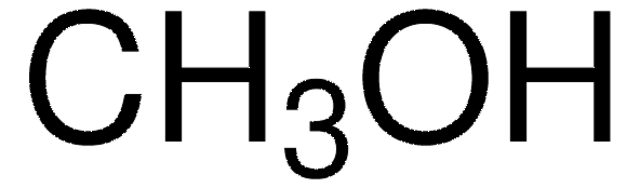RTC000087
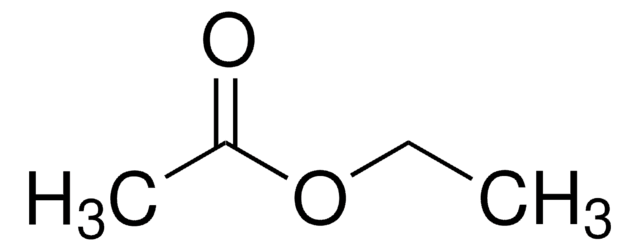
Ethyl Acetate
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Ethyl acetate, EtOAc
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to USP 1265402
vapor density
3 (20 °C, vs air)
vapor pressure
73 mmHg ( 20 °C)
CofA
current certificate can be downloaded
autoignition temp.
801 °F
expl. lim.
2.2-11.5 %, 38 °F
packaging
pkg of 20 mL
refractive index
n20/D 1.3720 (lit.)
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | 1265402 | 1.00864 | 1.09623 |
|---|---|---|---|
| application(s) pharmaceutical small molecule | application(s) pharmaceutical (small molecule) | application(s) liquid formulation | application(s) environmental |
| format neat | format neat | format neat | format neat |
| Quality Level 300 | Quality Level - | Quality Level 500 | Quality Level 300 |
| grade certified reference material, pharmaceutical secondary standard | grade pharmaceutical primary standard | grade - | grade ACS reagent, for analytical purposes, for extraction, reagent grade |
| storage temp. 2-30°C | storage temp. - | storage temp. 2-30°C | storage temp. 2-30°C |
| packaging pkg of 20 mL | packaging - | packaging - | packaging - |
General description
Analysis Note
Other Notes
Footnote
Recommended products
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
target_organs
Central nervous system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
26.6 °F
flash_point_c
-3 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service