56845
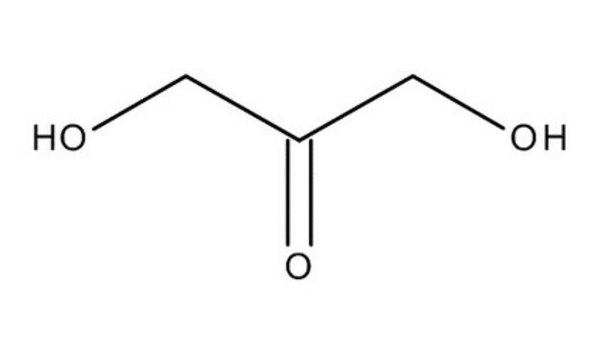
L-(+)-Erythrulose
≥75% (HPLC)
Synonym(s):
S-1,3,4-Trihydroxy-2-butanone, L-Glycero-2-tetrulose
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O4
CAS Number:
Molecular Weight:
120.10
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
assay
≥75% (HPLC)
form
liquid
optical activity
[α]/D 12.0±2.0°, c = 2 in H2O (24 h)
impurities
≤23% water
color
light yellow
storage temp.
room temp
SMILES string
OC[C@H](O)C(=O)CO
InChI
1S/C4H8O4/c5-1-3(7)4(8)2-6/h3,5-7H,1-2H2/t3-/m0/s1
InChI key
UQPHVQVXLPRNCX-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
Application
L-(+)-Erythrulose is used as a tanning agent in the cosmetics industry and a source of chiral ethyl ketones used in aldo reaction organic synthesis.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Santiago Díaz-Oltra et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(30), 9240-9254 (2008-08-30)
Both matched and mismatched diastereoselection have been observed in aldol reactions of a boron enolate of a protected L-erythrulose derivative with several chiral alpha-fluoro and alpha-amino aldehydes. Strict adherence to the Felkin-Anh model for the respective transition structures does not
Santiago Díaz-Oltra et al.
The Journal of organic chemistry, 70(20), 8130-8139 (2005-11-10)
[Chemical reaction: See text] Both matched and mismatched diastereoselections have been observed in the aldol reactions of a range of chiral aldehydes with the dicyclohexylboron enolate of a chiral ethyl ketone related to L-erythrulose. As was previously observed in the
J Alberto Marco et al.
The Journal of organic chemistry, 68(22), 8577-8582 (2003-10-25)
Both matched and mismatched diastereoselections have been observed in aldol reactions of the B,B-dicyclohexylboron enolate of a protected l-erythrulose derivative with a range of chiral aldehydes. The stereochemical outcome of reactions with alpha-methyl aldehydes can be adequately explained within the
G L Simpson et al.
Biochimica et biophysica acta, 1501(1), 12-24 (2000-03-23)
The degradation of L-ascorbate (AsA) and its primary oxidation products, L-dehydroascorbate (DHA) and 2,3-L-diketogulonate (2, 3-DKG) were studied under physiological conditions. Analysis determined that L-erythrulose (ERU) and oxalate were the primary degradation products of ASA regardless of which compound was
Xingxing Zou et al.
Journal of agricultural and food chemistry, 65(35), 7721-7725 (2017-07-15)
L-erythrose, a rare aldotetrose, possesses various pharmacological activities. However, efficient L-erythrose production is challenging. Currently, L-erythrose is produced by a two-step fermentation process from erythritol. Here, we describe a novel strategy for the production of L-erythrose in Gluconobacter oxydans (G.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service