D5782
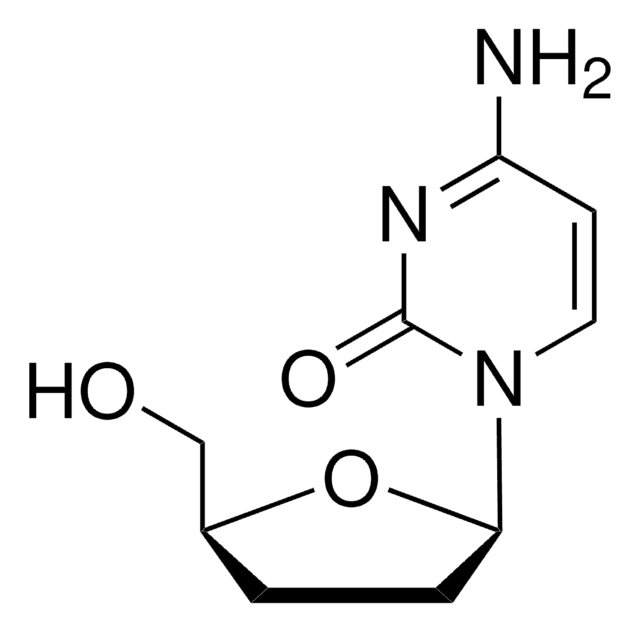
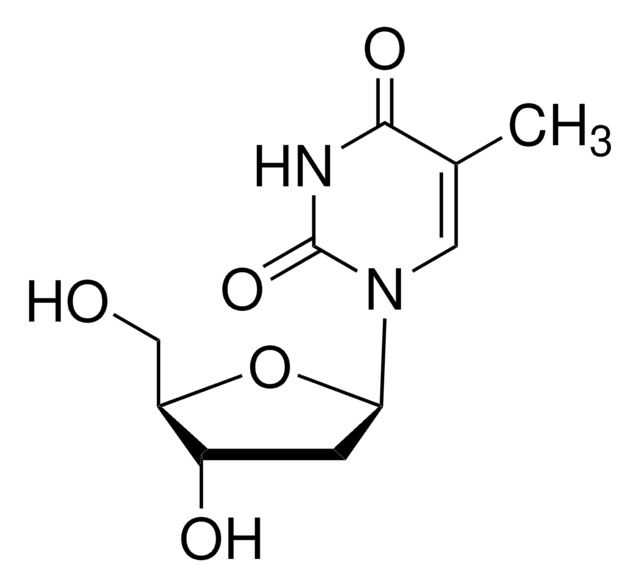
2′,3′-Dideoxycytidine
≥98% (HPLC)
Synonym(s):
ddC
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C9H13N3O3
CAS Number:
Molecular Weight:
211.22
Beilstein/REAXYS Number:
654956
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.52
Recommended Products
biological source
synthetic (organic)
Quality Level
assay
≥98% (HPLC)
form
powder
color
colorless
mp
217-218 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
NC1=NC(=O)N(C=C1)[C@H]2CC[C@@H](CO)O2
InChI
1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1
InChI key
WREGKURFCTUGRC-POYBYMJQSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2′,3′-Dideoxycytidine is used:
- as a DNA chain-terminating nucleotide for DNA sequencing methods based on the Sanger chain-termination method
- as a nucleoside reverse transcriptase inhibitor (NRTI) to study its effects on the development of mechanical allodynia in aging mice
- as a mitochondrial DNA (mtDNA) replication inhibitor to inhibit the activation of cGAS-STING pathway and study its effects on signaling protein-stimulator of interferon genes (STING), cyclic GMP-AMP synthase (cGAS), and phospho-interferon regulator factor 3 (p-IRF3) expression in mouse hippocampal and microglial cells
- as an NRTI inhibitor to study its effects on the drug induced-mitochondrial toxicity in Caenorhabditis elegans
Biochem/physiol Actions
2′,3′-Dideoxycytidine (ddC), is an ionic compound and a nucleoside analog. It acts as a nucleoside reverse transcriptase inhibitor and exhibits therapeutic effects against human immunodeficiency virus (HIV) infection. 2′,3′-Dideoxycytidine possesses anti-adenovirus activity and inhibits the adenovirus polymerase.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service