L1750
L-(+)-Lactic acid
≥98%
Synonym(s):
(S)-2-Hydroxypropionic acid, Sarcolactic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H6O3
CAS Number:
Molecular Weight:
90.08
Beilstein/REAXYS Number:
1720251
EC Number:
MDL number:
UNSPSC Code:
12161700
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
assay
≥98%
storage temp.
2-8°C
SMILES string
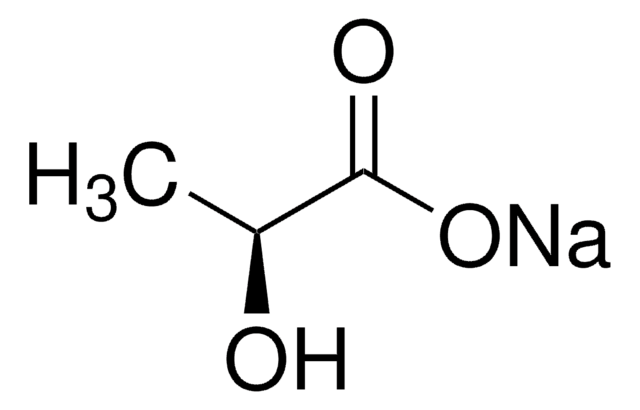
C[C@H](O)C(O)=O
InChI
1S/C3H6O3/c1-2(4)3(5)6/h2,4H,1H3,(H,5,6)/t2-/m0/s1
InChI key
JVTAAEKCZFNVCJ-REOHCLBHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
L-(+)-Lactic acid is the only naturally occurring lactic acid in humans and mammals. Commercially, few bacteria like Lactobacillus casei, L. delbrueckii, Streptococcus lactis produces L-Lactic acid by fermentation process. Lactic acid activates hydroxycarboxylic acid receptor, G-protein coupled receptor 81 (GPR81).
Application
Lactic acid has been used:
- as a component in substrate solution II for lactate dehydrogenase reaction
- as an additive in storage solution A
- as a supplement in the artificial gastric juice preparation for evaluation of degree of resistance Lactobacillus to the gastric stresses
Biochem/physiol Actions
L-(+)-Lactic acid is used as a substrate for lactic acid dehydrogenase and lactate oxidase.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1C
supp_hazards
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
230.0 °F - closed cup
flash_point_c
110.00 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service