L7391
Anti-LIS1 antibody, Mouse monoclonal
clone LIS1-338, purified from hybridoma cell culture
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Recommended Products
biological source
mouse
Quality Level
conjugate
unconjugated
antibody form
purified from hybridoma cell culture
antibody product type
primary antibodies
clone
LIS1-338, monoclonal
form
buffered aqueous solution
mol wt
antigen 46 kDa
species reactivity
zebrafish, chicken, rat, human, bovine, mouse
concentration
~2 mg/mL
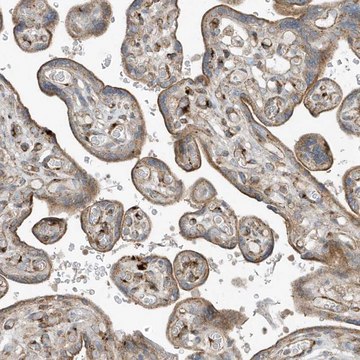
General description
Monoclonal Anti-LIS1 (mouse IgG1 isotype) is derived from the LIS1-338 hybridoma produced by the fusion of mouse myeloma cells and splenocytes from a BALB/c mouse immunized with a recombinant human LIS1 protein. The LIS1 protein (46 kDa) contains seven WD (tryptophan-aspartic acid) repeats, a motif shared by at least 140 known proteins.
The gene PAFAH1B1 (platelet activating factor acetylhydrolase 1b regulatory subunit 1) encodes a protein named LIS1 (lissencephaly-1) that forms the α subunit of the intracellular Ib isoform of platelet-activating factor acteylhydrolase (PAFAH). The gene is mapped to human chromosome 17p13.3.
Specificity
By immunoblotting, the antibody prefers the phosphorylated form of the LIS1 molecule (46 kDa).
Immunogen
recombinant human LIS1 protein.
Application
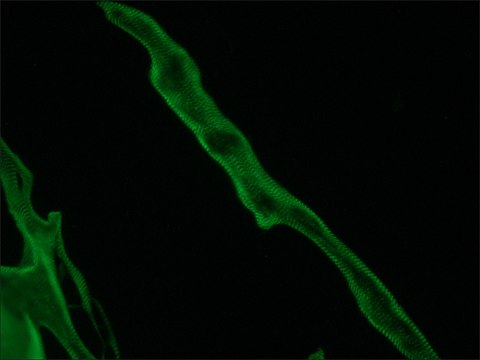
Monoclonal Anti-LIS1 antibody produced in mouse has been used in protein pulldown assay and immunofluorescence.
Biochem/physiol Actions
LIS1 is involved in cell regulation, including the β subunits of G-proteins (Gβ). This protein motif is likely to mediate protein-protein interactions. LIS1 is a protein that is highly conserved during evolution; bovine, mouse, and chicken proteins exceed 99% similarity.
The heterotrimeric enzyme, platelet-activating factor acteylhydrolase (PAFAH), catalyzes the cleavage of acetyl group at the SN-2 position of platelet-activating factor. The α subunit encoded by the gene PAFAH1B1 (platelet activating factor acetylhydrolase 1b regulatory subunit 1) interacts with tubulin and affects microtubule dynamics. Mutations in this gene have been linked to Miller–Dieker lissencephaly, a human brain malformation characterized by a smooth cerebral surface and a disordered organization of the cortical layers resulting from a defect in neuronal migration.
Physical form
Solution in 0.01 M phosphate buffered saline, pH 7.4, containing 15 mM sodium azide.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class
10 - Combustible liquids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service