M6626
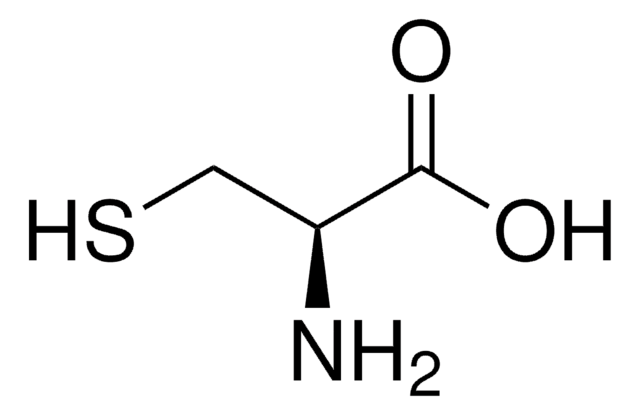
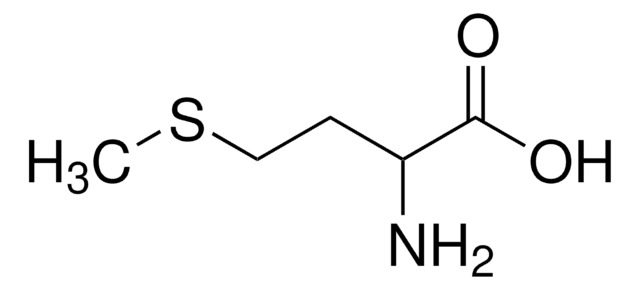
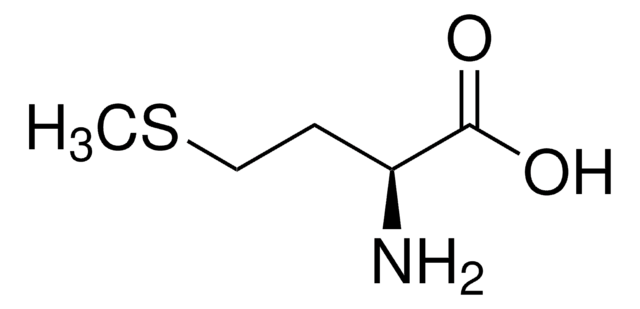
S-Methyl-L-cysteine
suitable for ligand binding assays
Synonym(s):
(R)-2-Amino-3-(methylmercapto)propionic acid, SMLC
About This Item
Recommended Products
Product Name
S-Methyl-L-cysteine, substrate for methionine sulfoxide reductase A
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
CSC[C@H](N)C(O)=O
InChI
1S/C4H9NO2S/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1
InChI key
IDIDJDIHTAOVLG-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
Application
- In-vitro antioxidant and anti-inflammatory potential along with p.o. pharmacokinetic profile of key bioactive phytocompounds of Snow Mountain Garlic: a comparative analysis vis-à-vis normal garlic.: This study examines the antioxidant and anti-inflammatory properties of S-Methyl-L-cysteine found in Snow Mountain Garlic, providing insights into its potential therapeutic applications (Kaur et al., 2024).
- Acidification and tissue disruption affect glucosinolate and S-methyl-l-cysteine sulfoxide hydrolysis and formation of amines, isothiocyanates and other organosulfur compounds in red cabbage (Brassica oleracea var. capitata f. rubra).: The research highlights how acidification and tissue disruption influence the hydrolysis of S-Methyl-L-cysteine sulfoxide, impacting the formation of various bioactive compounds in red cabbage (Hanschen, 2024).
- Selective cycloaddition of ethylene oxide to CO(2) within the confined space of an amino acid-based metal-organic framework.: This research explores the use of an S-Methyl-L-cysteine-based metal-organic framework for the selective cycloaddition of ethylene oxide to CO2, demonstrating its potential in green chemistry and materials science (Bilanin et al., 2023).
Biochem/physiol Actions
When used as a dietary supplement in the Drosphilia model of PD, SMLC increases the efficacy of the MRSA catalytic antioxidant system by providing additional substrate available leading to increased resistance to oxidative stress.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service