P7130
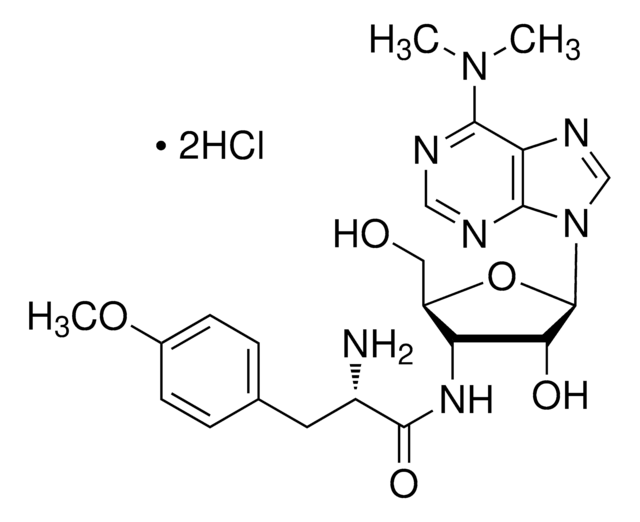
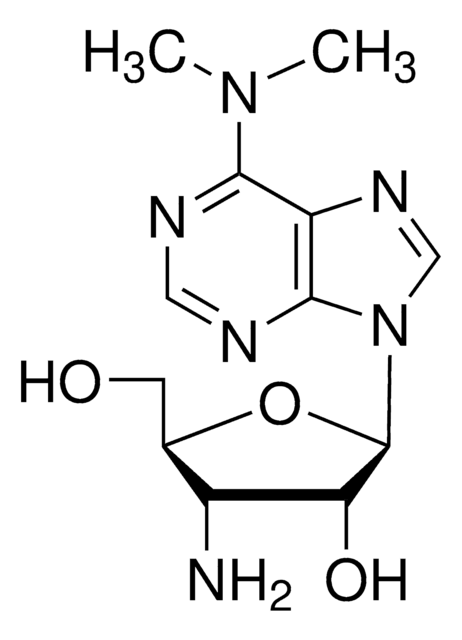
Puromycin aminonucleoside
Synonym(s):
3′-Amino-3′-deoxy-N6,N6-dimethyladenosine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C12H18N6O3
CAS Number:
Molecular Weight:
294.31
Beilstein/REAXYS Number:
93902
EC Number:
MDL number:
UNSPSC Code:
51281912
PubChem Substance ID:
NACRES:
NA.85
Recommended Products
form
powder
Quality Level
solubility
H2O: soluble 50 mg/mL
antibiotic activity spectrum
Gram-positive bacteria
neoplastics
parasites
mode of action
protein synthesis | interferes
storage temp.
2-8°C
SMILES string
CN(C)c1ncnc2n(cnc12)[C@@H]3O[C@H](CO)[C@H](N)[C@@H]3O
InChI
1S/C12H18N6O3/c1-17(2)10-8-11(15-4-14-10)18(5-16-8)12-9(20)7(13)6(3-19)21-12/h4-7,9,12,19-20H,3,13H2,1-2H3/t6-,7+,9+,12-/m1/s1
InChI key
RYSMHWILUNYBFW-VENHTOENSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
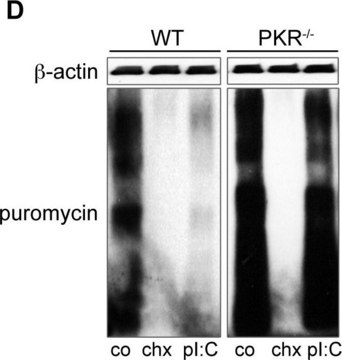
Puromycin aminonucleoside is the aminonucleoside portion of the antibiotic puromycin. It is useful in nephrology research, like studies of focal and segmental glomerulosclerosis and in the induction of nephrosis in rats. The excretion of sodium and nitrite (NOx) metabolites in rats with puromycin aminonucleoside-induced nephrotic syndrome are studied. Puromycin aminonucleoside-induced nephrosis in rats has been studied with respect to the production of reactive oxygen species during the acute phase. Puromycin aminonucleoside is used to probe endothelial glycosaminoglycan synthesis in cultured glomerular endothelial cells and their relation to cell permeability.
Application
Puromycin aminonucleoside has been used:
- as a selection marker for the infected cells for the transfection of lentivirus
- as a component of fresh medium for transfection and infection assays
- to subcutaneously induce puromycin aminonucleoside nephrosis to investigate the mRNA and protein levels of nephrin and podocin before the onset of proteinuria
Biochem/physiol Actions
Puromycin aminonucleoside is used to study human glomerular disease by inducing damage of murine glomerular podocytes and is used to study glomerular function and morphology.
Other Notes
Keep container tightly closed in a dry and well-ventilated place.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service