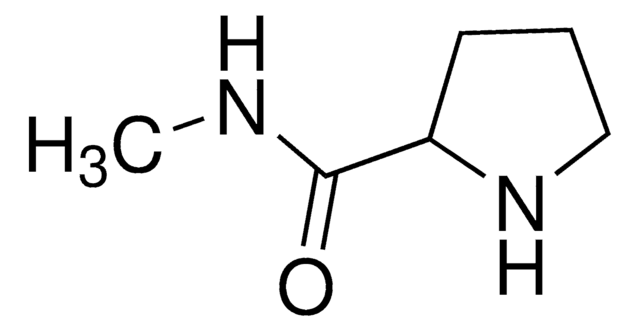
U211140
DMT-2′O-Methyl-rU Phosphoramidite
configured for MerMade
Synonym(s):
5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-methyl-uridine, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], DMT-2′O-Methyl-rU amidite
Select a Size
Select a Size
About This Item
Recommended Products
biological source
non-animal source (no BSE/TSE risk)
Quality Level
product line
Proligo Reagents
assay
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
form
powder
mol wt
760.81 g/mol
impurities
≤0.3% mU2 (reversed phase HPLC, Hydrolysate)
≤0.3% mU3 (reversed phase HPLC, DMT-rme)
≤0.3% water content (Karl Fischer)
≤0.5% P(III) Impurities 100-169ppm (31P-NMR)
≤0.5% single Impurity (redox titration)
≤1.0% mU1 (reversed phase HPLC, DMT-rUme-DMT)
≤3% residual Solvent content
color
white to off-white
suitability
conforms to structure for H-NMR
conforms to structure for LC-MS
compatibility
configured for MerMade
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
- High yield of crude oligonucleotides Compatible with DNA synthesis
- Can be employed together with DNA or RNA phosphoramidites in the same synthesis to produce mixmer oligonucleotides
- Recommended deprotection conditions are 8 hours at 55 deg C using concentrated ammonia solution, or with AMA (concentrated ammonia/40% aqueous methylamine I/I, v/v) for 10 minutes at 65 deg C
- Purification and other downstream processing of fully modified 2′OMethyl RNA oligonucleotides are simpler than in the case of RNA, as no special precautions are required to provide protection against nucleolytic degradation
- Synthesis of 2′O-Methyl RNA oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 6minutes compared to 90 seconds for DNA monomers)
- 2′O-Methyl RNA phosphoramidites are also available with fast deprotection chemistry
Application
- Diagnostic probes
- Aptamer and ribozyme development
- Mixed 2′O-Methyl-RNA/DNA antisense molecules
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service