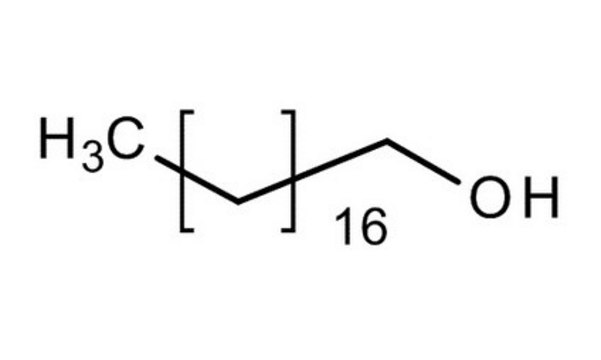
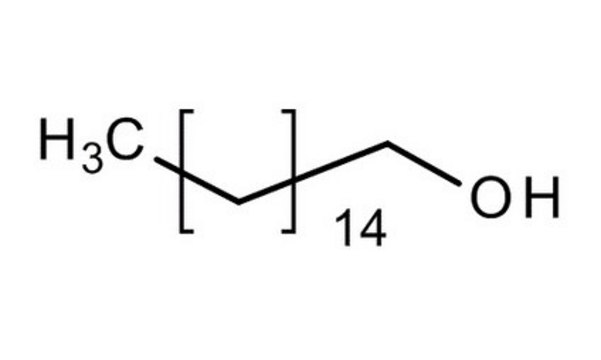
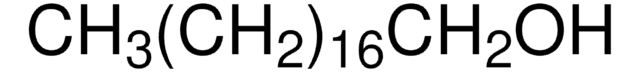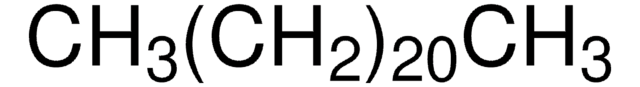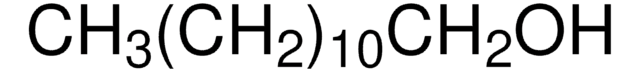O709
1-Octadecanol
95%
Synonym(s):
Octadecyl alcohol, Stearyl alcohol
Select a Size
About This Item
Recommended Products
vapor density
9.3 (vs air)
Quality Level
vapor pressure
<0.01 mmHg ( 38 °C)
Assay
95%
form
solid
autoignition temp.
842 °F
expl. lim.
~8 %
bp
210 °C/15 mmHg
mp
56-59 °C (lit.)
solubility
H2O: slightly soluble 0.001 g/L at 23 °C
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 258768 | 74723 | 8.07680 |
|---|---|---|---|
| assay 95% | assay 99% | assay ≥99.0% (GC) | assay ≥96.0% (GC) |
| solubility H2O: slightly soluble 0.001 g/L at 23 °C | solubility water: slightly soluble 0.001 g/L at 23 °C | solubility THF: 1 g/10 mL, clear, colorless | solubility - |
| Quality Level 200 | Quality Level 200 | Quality Level 200 | Quality Level 200 |
| bp 210 °C/15 mmHg | bp 170-171 °C/2 mmHg | bp - | bp 330-360 °C/1013 hPa |
| form solid | form - | form pellets | form scales |
| density 0.812 g/mL at 25 °C (lit.) | density 0.812 g/mL at 25 °C (lit.) | density 0.812 g/mL at 25 °C (lit.) | density 0.805-0.815 g/cm3 at 60 °C |
Application
- 1-Octadecanol is used in the synthesis of O-octadecyl-S-trifluorothiolcarbonate, which is a storable crystalline source of trifluoromethanethiol.[1]
- It can be used to improve solution processability of conjugated polymers used in the organic electronic applications.[2]
- It induces hydrophobicity to the amphiphiles used in the drug and gene delivery.[3][4]
- It is also employed in the synthesis of solid-liquid phase change materials (PCMs)[5][6], surfactant and stabilizers.[7]
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
383.0 °F - closed cup
Flash Point(C)
195 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service