M0375
Maleic acid
ReagentPlus®, ≥99% (HPLC)
Synonym(s):
cis-Butenedioic acid, Toxilic acid
Sign Into View Organizational & Contract Pricing
About This Item
Linear Formula:
HO2CCH=CHCO2H
CAS Number:
Molecular Weight:
116.07
Beilstein:
605762
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Pricing and availability is not currently available.
Recommended Products
grade
reagent grade
Quality Level
vapor density
4.0 (at °C, vs air)
vapor pressure
≤0.1 at hPa ( 20 °C)
product line
ReagentPlus®
Assay
≥99% (HPLC)
form
powder
concentration
≤100%
impurities
≤2.0% water
ign. residue
≤0.1%
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Maleic acid (MA) is a dicarboxylic acid that undergoes esterification with ethanol in the presence of cation-exchange resin catalysts to form diethyl maleate.[1] Its crystalline structure has been analyzed.[2] Reaction kinetics and mechanism of the isomerization of maleic acid to fumaric acid in the presence of ceric and bromide ions has been investigated.[3] The reaction mechanism and reactivity ratios for the radical copolymerization of maleic acid with styrene have been determined.[4]
Application
Maleic acid has been used in the preparation of tris-maleate buffer[5] and sodium maleate buffer.[6][7] It has also been used for the pretreatment of poplar tracheid cell walls for the spectroscopic analysis of lignin.[8] It may be used to synthesize superswelling acrylamide/maleic acid hydrogels.[9]
Analysis Note
Repurified
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A novel USP9X substrate TTK contributes to tumorigenesis in non-small-cell lung cancer.
Chen X, et al.
Theranostics, 8(9), 2348-2348 (2018)
O-GlcNAcylation of fumarase maintains tumour growth under glucose deficiency
Wang T, et al.
Nature Cell Biology, 19(7), 833-833 (2017)
The FLAG? peptide, a versatile fusion tag for the purification of recombinant proteins.
Einhauer A and Jungbauer A
Journal of Biochemical and Biophysical Methods, 49(1-3), 455-465 (2001)
Local generation of fumarate promotes DNA repair through inhibition of histone H3 demethylation
Jiang Y, et al.
Nature Cell Biology, 17(9), 1158-1158 (2015)
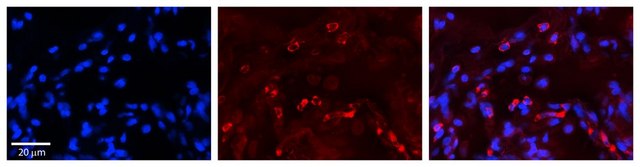
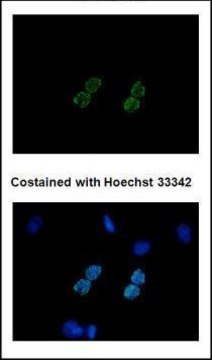
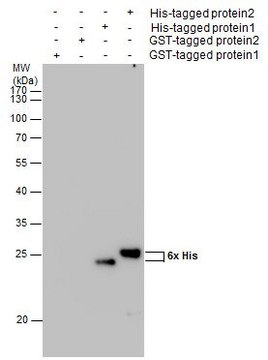
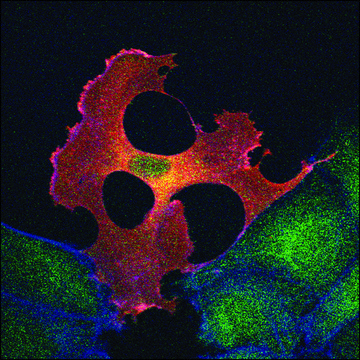
Ningning Shi et al.
Advanced biology, 6(11), e2200092-e2200092 (2022-07-13)
Site-specific incorporation of distinct noncanonical amino acids (ncAAs) into proteins via genetic code expansion in mammalian cells represents a new avenue for protein engineering. Reassigning three TAGs with the same ncAA in mammalian cells has previously been achieved using translational
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service