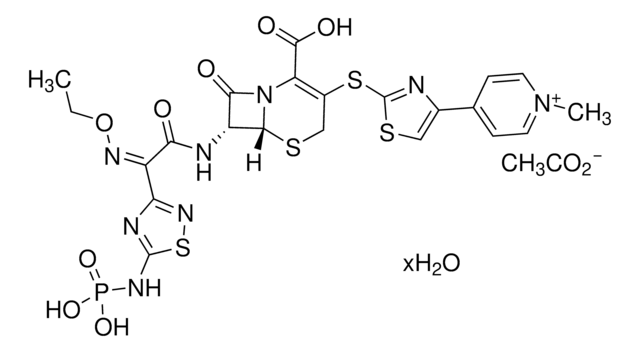
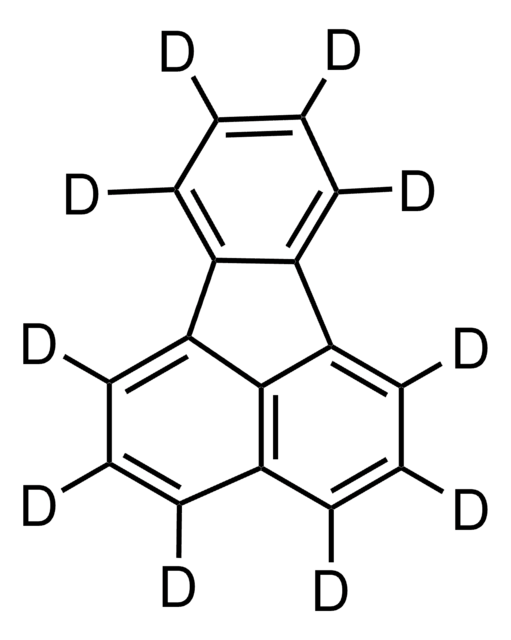
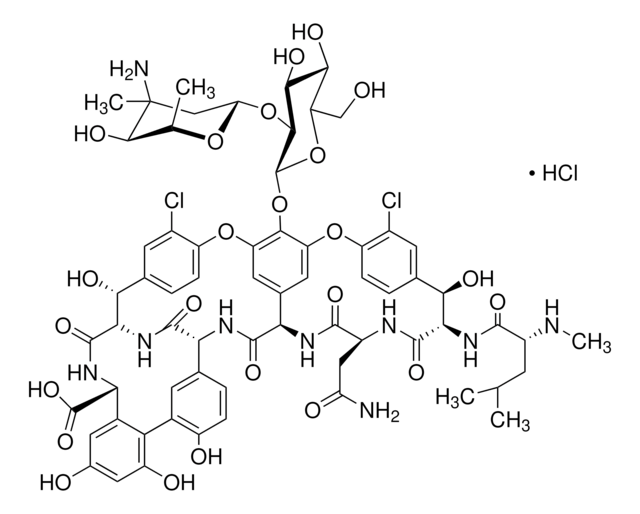
TC-50L-BK
Blood typing Bulk Reagent Diluents - ABO & RHD (Rh) Specificities
ABO Reagent Diluent
Synonyme(s) :
ABO Reagent Diluent
About This Item
Produits recommandés
Forme d'anticorps
unpurified
Niveau de qualité
Forme
liquid
Conditionnement
pack of 50 L
Concentration
8-12 mg/mL protein (Total Protein Range)
Technique(s)
agglutination assay: suitable
pH
7.1-7.3
Conditions d'expédition
ambient
Température de stockage
2-8°C
Catégories apparentées
1 of 4
Cet article | 364622 | 456292 | 364630 |
|---|---|---|---|
| isotopic purity 98 atom % D | isotopic purity 98 atom % D | isotopic purity 98 atom % D | isotopic purity 98 atom % D |
| mass shift M+10 | mass shift M+10 | mass shift M+10 | mass shift M+14 |
| Quality Level 200 | Quality Level 200 | Quality Level 200 | Quality Level 200 |
| form solid | form solid | form solid | form solid |
| mp 210-215 °C (lit.) | mp 98-100 °C (lit.) | mp 110-113 °C (lit.) | mp 212-213 °C (lit.) |
| bp 340 °C (lit.) | bp 340 °C (lit.) | bp 384 °C (lit.) | bp - |
Description générale
Filtered through a 0.22μm terminal filter
Shelf Life: 36 months from date of filtration
Product formulation examined according to Annex IV of the EU IVD directive to support CE registration.
For In Vitro use only
Application
Informations légales
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique






![GAL4 [(1-147) + VP16 (411-490)] from Saccharomyces cerevisiae human herpesvirus 2 recombinant, expressed in E. coli, ≥80% (SDS-PAGE)](/deepweb/assets/sigmaaldrich/product/images/195/570/27f4c1ab-cab8-46e7-bd1a-04978b89bacc/640/27f4c1ab-cab8-46e7-bd1a-04978b89bacc.jpg)