G3664
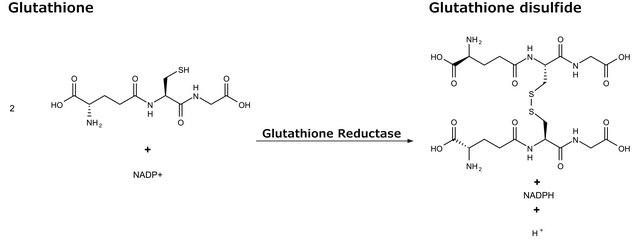
Glutathione Reductase from baker′s yeast (S. cerevisiae)
ammonium sulfate suspension, 100-300 units/mg protein (biuret)
Synonyme(s) :
GR, NAD(P)H:oxidized-glutathione oxidoreductase
About This Item
Produits recommandés
Forme
ammonium sulfate suspension
Niveau de qualité
Activité spécifique
100-300 units/mg protein (biuret)
Poids mol.
118 kDa
Numéro d'accès UniProt
Activité étrangère
G-6-PDH, 6-PGDH, and NADPH oxidase ≤0.01%
lipoamide dehydrogenase ≤0.1%
Température de stockage
2-8°C
Informations sur le gène
bakers yeast ... GLR1(856014)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
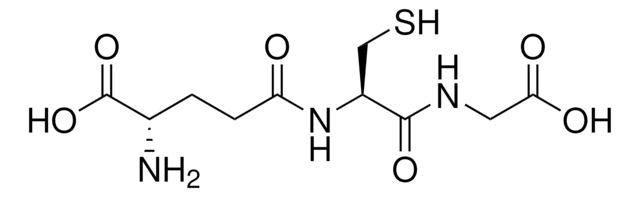
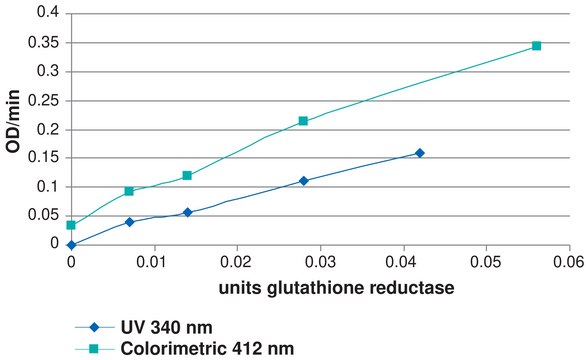
- in the glutathione assay to determine glutathione concentration.
- as a standard in the generation of calibration curve.
- as an antigen to measure plasma activity of GR.
- for quantifying the myocardial tissue glutathione content using a glutathione reductase-5,5′-dithiobis (2-nitrobenzoic acid)-based enzymatic recycling assay
- for the quantification of reduced glutathione (GSH) in the oocytes, using a slightly modified microglutathione assay, obtained from prepubertal gilts
- for the preparation of total GSSG (glutathione disulphide) + GSH measurement, where all available GSSG was reduced to GSH, in rat lens
- for the quantification of intracellular reduced glutathione (GSH) in the oocytes obtained from rats
Actions biochimiques/physiologiques
Définition de l'unité
Forme physique
Notes préparatoires
Inhibiteur
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique