227129
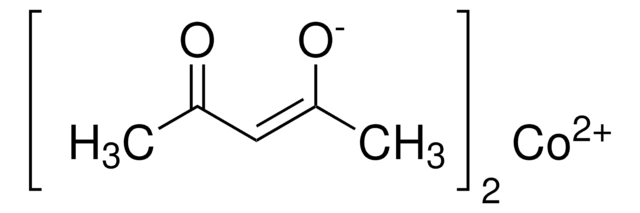
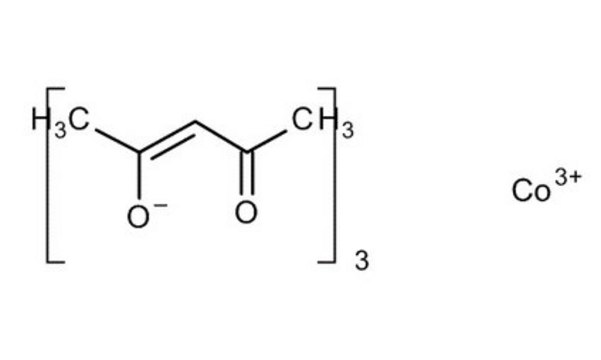
Cobalt(II) acetylacetonate
97%
동의어(들):
2,4-Pentanedione cobalt(II) derivative, Bis(2,4-pentanedionato)cobalt, Co(acac)2, Cobaltous acetylacetonate
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
Co(C5H7O2)2
CAS Number:
Molecular Weight:
257.15
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
Quality Level
분석
97%
양식
powder and chunks
반응 적합성
core: cobalt
불순물
≤3% water
mp
165-170 °C (lit.)
SMILES string
CC(=O)\C=C(\C)O[Co]O\C(C)=C/C(C)=O
InChI
1S/2C5H8O2.Co/c2*1-4(6)3-5(2)7;/h2*3,6H,1-2H3;/q;;+2/p-2/b2*4-3-;
InChI key
UTYYEGLZLFAFDI-FDGPNNRMSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Cobalt(II)acetylacetonate (Co(acac)2) is a coordination compound in which acentral cobalt atom is coordinated to two acetylacetonate ligands, which act asbidentate ligands. It is used in various applications such as organicsynthesis, coordination chemistry, and as a catalyst in several chemicalreactions. Additionally, Co(acac)2 is used as a precursor in processes such aschemical vapor deposition (CVD) and laser evaporation techniques for thefabrication of thin films. It also plays a crucial role in the production ofcobalt oxide thin films, which find applications in electronics, catalysis, andother fields requiring thin film coatings.
애플리케이션
- Cobalt (II)-Catalyzed Isocyanide Insertion Reaction with Amines: Details a synthetic method for forming ureas and azaheterocycles catalyzed by Cobalt(II) acetylacetonate, applicable in pharmaceutical synthesis (Zhu et al., 2014).
- Cobalt‐Catalyzed C−H Functionalizations by Imidate Assistance: Describes a method using Cobalt(II) acetylacetonate for C-H functionalization, important for organic synthesis and material chemistry (Mei & Ackermann, 2016).
- Cobalt (II) acetylacetonate covalently anchored onto magnetic mesoporous silica nanospheres: Focuses on its use as a catalyst for epoxidation of olefins, relevant for catalysis research (Li et al., 2015).
Cobalt(II) acetylacetonate can be used:
- A precursor in the solvothermal synthesis of Co3O4 nanoparticles. These nanoparticles exhibit high electrochemical performance and are used as a potential supercapacitor material due to their excellent capacitance and cycling stability.
- A precursor in the preparation of Co3O4 nanoparticles via hydrothermal method. The resulting Co3O4 nanoparticles exhibit a highly-uniform mesoporous structure and tunable sizes, making them promising for CO sensing applications.
- A precursor for the growth of cobalt oxide thin films using Metal-Organic Chemical Vapor Deposition (MOCVD).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
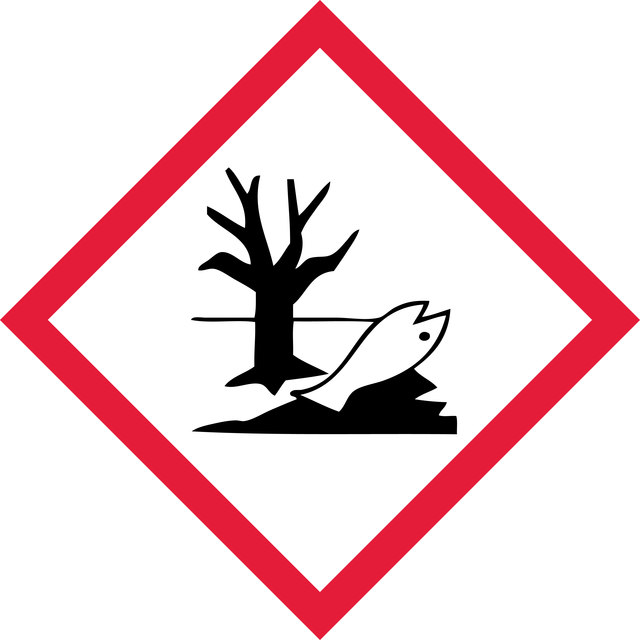
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.