추천 제품
Quality Level
분석
98%
양식
solid
bp
264 °C (lit.)
mp
114-120 °C (lit.)
SMILES string
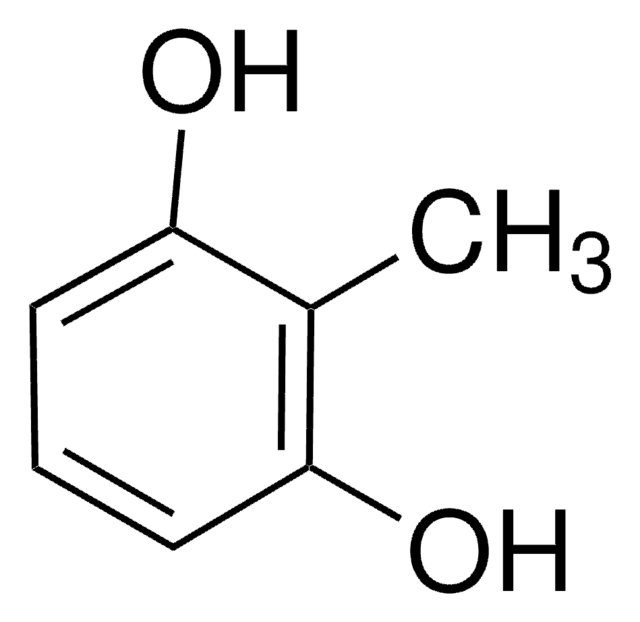
Cc1c(O)cccc1O
InChI
1S/C7H8O2/c1-5-6(8)3-2-4-7(5)9/h2-4,8-9H,1H3
InChI key
ZTMADXFOCUXMJE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The reaction between 2-methylresorcinol and 2-alkenals was studied to investigate the scavenging ability of m-diphenols for the 2-alkenals formed during lipid oxidation.
애플리케이션
2-Methylresorcinol was used in the synthesis of:
- C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene
- tripyrrane analogs
- series of novel aromatic benziporphyrins
신호어
Danger
유해 및 위험 성명서
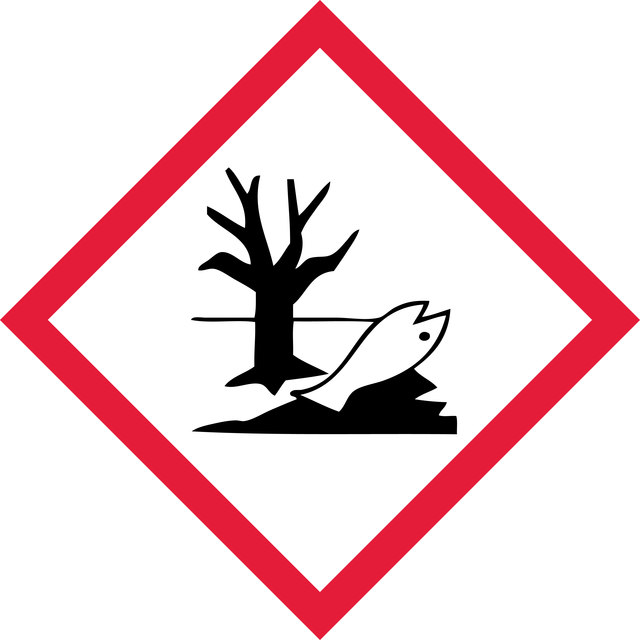
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
275.0 °F - closed cup
Flash Point (°C)
135 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Kae Miyake et al.
Chemical communications (Cambridge, England), (2)(2), 178-179 (2004-01-23)
Acid catalyzed condensation of resorcinol or 2-methylresorcinol with 2 equiv. of an acetoxymethylpyrrole gave bis(pyrrolylmethyl)benzene derivatives in moderate yields; these afforded a series of novel aromatic benziporphyrins using the MacDonald "3 + 1" methodology.
Hamza M Abosadiya et al.
Molecules (Basel, Switzerland), 18(11), 13369-13384 (2013-11-01)
C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene (I) was synthesized by cyclocondensation of 5-bromo-2-hydroxybenzaldehyde and 2-methylresorcinol in the presence of concentrated HCl. Compound I was characterized by infrared and nuclear magnetic resonance spectroscopic data. X-ray analysis showed that this compound crystallized in a triclinic system with
Francisco J Hidalgo et al.
Food chemistry, 160, 118-126 (2014-05-07)
The reaction between m-diphenols (resorcinol, 2-methylresorcinol, 2,5-dimethylresorcinol, 3-methylphenol, orcinol, and phloroglucinol) and 2-alkenals (2-pentenal and 2-octenal) was studied in an attempt to understand the chemical pathways involved in the scavenging ability of m-diphenols for the 2-alkenals produced as a consequence
Timothy D Lash et al.
The Journal of organic chemistry, 76(15), 6295-6308 (2011-06-23)
Tripyrrane analogues were prepared by reacting resorcinol or 2-methylresorcinol with 2 equiv of an acetoxymethylpyrrole in the presence of p-toluenesulfonic acid and calcium chloride. Following removal of the benzyl ester protective groups, the resorcinol-derived benzitripyrrane was reacted with a pyrrole
Hye Jung Choi et al.
Journal of microbiology and biotechnology, 22(9), 1214-1217 (2012-07-21)
One Helicosporium strain, isolated from a wilted chestnut tree, evidenced in vitro antimicrobial activity against various types of bacteria and fungi, and generated a diffusible pigment. The antimicrobial compounds and the diffusible pigment of the Helicosporium sp. isolate were purified
프로토콜
Separation of Resorcinol 50 mg/mL; Pyrocatechol; 2-Methylresorcinol; 4-Methylcatechol; 2,5-Dimethylresorcinol 50 mg/mL; 3-Methylcatechol 50 mg/mL; 4-Nitrocatechol 50 mg/mL
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.