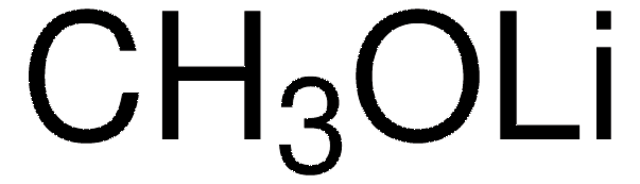추천 제품
Quality Level
농도
~10% in methanol (T, ~2.2 M)
density
0.85 g/mL at 20 °C
SMILES string
[Li+].C[O-]
InChI
1S/CH3O.Li/c1-2;/h1H3;/q-1;+1
InChI key
JILPJDVXYVTZDQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Lithium methoxide solution is a useful reagent in organic synthesis with a range of applications. For example, it is used as a strong base in deprotonation reactions of various acidic functional groups, such as alcohols, phenols, and carboxylic acids. It also acts as a catalyst in esterification reactions. It can catalyze the reaction of carboxylic acids with alcohols to form esters.
애플리케이션
Lithium methoxide solution (10% in methanol) can be used as a source of lithium ions in the synthesis of dilithium (2,3-dilithium-oxy)-terephthalate, which is studied for its electrochemical behavior.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Self-heat. 1 - Skin Corr. 1B - STOT SE 1
표적 기관
Eyes,Central nervous system
보충제 위험성
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 2
Flash Point (°F)
53.6 °F
Flash Point (°C)
12 °C
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.