P8688
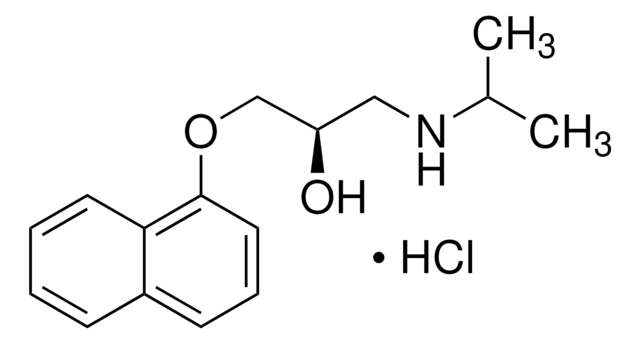
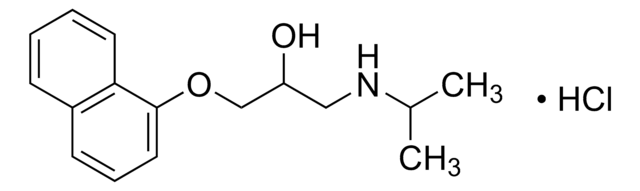
(S)-(−)-Propranolol hydrochloride
≥98% (TLC), powder
동의어(들):
(S)-1-Isopropylamino-3-(1-naphthyloxy)-2-propanol hydrochloride
About This Item
추천 제품
Quality Level
분석
≥98% (TLC)
양식
powder
광학 활성
[α]25/D −25.5°, c = 1.0 in ethanol(lit.)
mp
193-195 °C (lit.)
solubility
ethanol: 10 mg/mL
DMSO: <14.5 mg/mL
H2O: 50 mg/mL
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 8.0 mg/mL
주관자
AstraZeneca
저장 온도
2-8°C
SMILES string
Cl[H].CC(C)NC[C@H](O)COc1cccc2ccccc12
InChI
1S/C16H21NO2.ClH/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16;/h3-9,12,14,17-18H,10-11H2,1-2H3;1H/t14-;/m0./s1
InChI key
ZMRUPTIKESYGQW-UQKRIMTDSA-N
유전자 정보
human ... ADRB1(153), ADRB2(154), ADRB3(155), HTR1A(3350), HTR1B(3351), HTR1D(3352), HTR1E(3354), HTR1F(3355)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
애플리케이션
- as a non-selective β-blocker propranolol to inhibit the actions of epinephrine in mice
- as a β1- and β2-aadrenergic receptor blocker in rat
- as a medium supplement to investigate its effect on adipogenesis in hemangioma-derived stem cells (HemSC)
생화학적/생리학적 작용
특징 및 장점
애플리케이션
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.