C6154
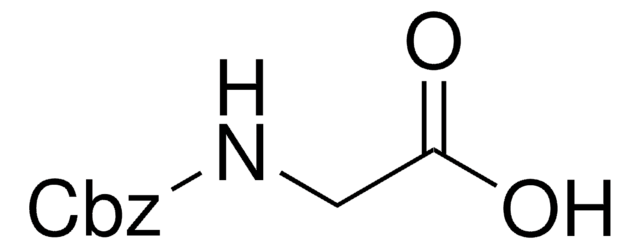
Z-Gln-Gly
γ-glutamyl donor substrate
Synonym(s):
N2-[(phenylmethoxy)carbonyl]-L-glutaminyl-glycine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H19N3O6
CAS Number:
Molecular Weight:
337.33
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
form
powder
storage temp.
−20°C
SMILES string
NC(=O)CCC(NC(=O)OCc1ccccc1)C(=O)NCC(O)=O
InChI
1S/C15H19N3O6/c16-12(19)7-6-11(14(22)17-8-13(20)21)18-15(23)24-9-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H2,16,19)(H,17,22)(H,18,23)(H,20,21)
InChI key
SOUXAAOTONMPRY-UHFFFAOYSA-N
Amino Acid Sequence
Z-Gln-Gly
Application
γ-Glutamyl donor substrate used in spectrophotometric determination of transglutaminase (TGase) activity. Z-Gln-Gly was used to enzymatically synthesize N-linked neoglycoproteins.
Biochem/physiol Actions
N-Benzyloxycarbonyl-L-Glutaminylglycine (Z-Gln-Gly, Z-QG) is used as a substrate to differentiate and characterize transglutaminase(s) (TGase) that catalyzes the post-translational covalent cross-linking of Gln- and Lys-containing peptides. Z-QG supports glutamyl-level cross-linking applications thruough surface modification.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service