All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H10N2O3
CAS Number:
Molecular Weight:
218.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
189-193 °C (lit.)
functional group
ester
SMILES string
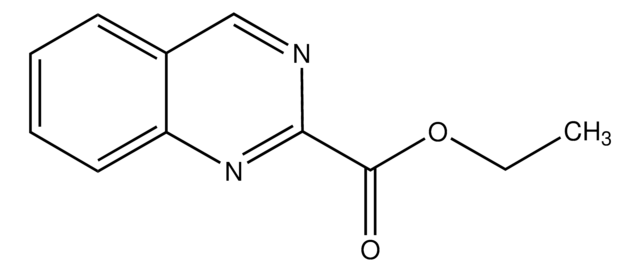
CCOC(=O)C1=Nc2ccccc2C(=O)N1
InChI
1S/C11H10N2O3/c1-2-16-11(15)9-12-8-6-4-3-5-7(8)10(14)13-9/h3-6H,2H2,1H3,(H,12,13,14)
InChI key
BMCAWNQKVVTNFP-UHFFFAOYSA-N
General description
Ethyl 4-quinazolone-2-carboxylate is a quinazoline derivative. It can be synthesized from anthranilamide and ethyl oxalate.
Application
Ethyl 4-quinazolone-2-carboxylate may be used to synthesize 4-quinazolone-2-carboxylic acid.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nonclassical Antimetabolites. X. 1, 2 A Facile Synthesis of 4-Quinazolone-2-carboxylic Acid and the Structure of Bogert's Ammonium Salt.
Baker BR and Almaula PI.
The Journal of Organic Chemistry, 27(12), 4672-4674 (1962)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service