About This Item
Recommended Products
vapor density
2.5 (vs air)
Quality Level
vapor pressure
90 mmHg ( 20 °C)
assay
≥99.0%
autoignition temp.
390 °F
expl. lim.
12.5 %
impurities
≤0.30% (water)
refractive index
n20/D 1.380 (lit.)
bp
75 °C (lit.)
mp
−96 °C (lit.)
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | P4338 | 81227 | 81189 |
|---|---|---|---|
| form waxy solid | form powder | form solid | form solid |
| Quality Level 200 | Quality Level 200 | Quality Level 200 | Quality Level 100, 200 |
| mol wt 3,000-3,700 | mol wt average mol wt 3,350 | mol wt Mr 2700-3300 | mol wt Mr 950-1050 |
| impurities endotoxin, tested | impurities ≤0.01% Phosphorus (P), ≤0.1% Insoluble matter | impurities insoluble matter, passes filter test, ≤0.001% peroxides (as H2O2) | impurities DNases, none detected, RNases, none detected, insoluble matter, passes filter test, phosphatases, none detected, proteases, none detected |
| biological source synthetic (organic) | biological source - | biological source - | biological source - |
| grade Hybri-Max™ | grade - | grade - | grade for molecular biology |
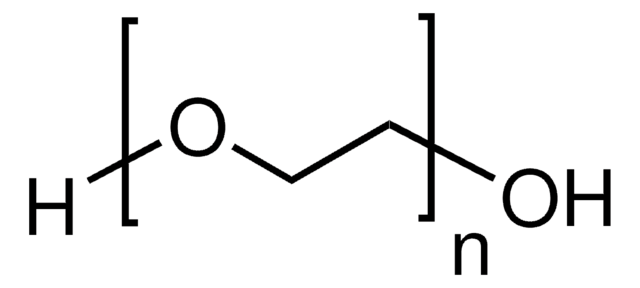
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
<50.0 °F - Pensky-Martens closed cup
flash_point_c
< 10 °C - Pensky-Martens closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service