P7754
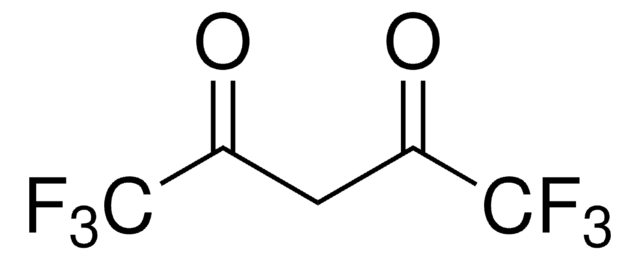
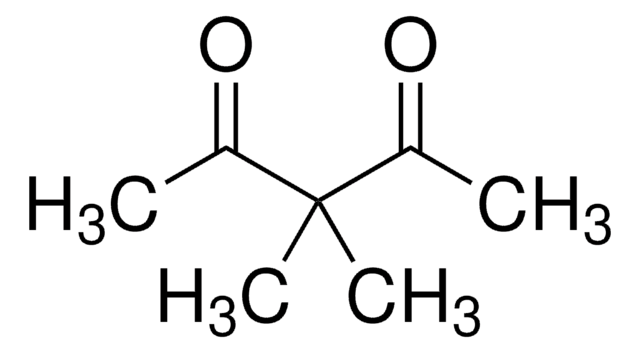
Acetylacetone
ReagentPlus®, ≥99%
Synonym(s):
2,4-Pentanedione
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(3)
Select a Size
Change View
About This Item
Linear Formula:
CH3COCH2COCH3
CAS Number:
Molecular Weight:
100.12
Beilstein/REAXYS Number:
741937
EC Number:
MDL number:
UNSPSC Code:
12352115
eCl@ss:
39021208
PubChem Substance ID:
NACRES:
NA.21
assay:
≥99%
bp:
140.4 °C (lit.)
vapor pressure:
6 mmHg ( 20 °C)
Recommended Products
vapor density
3.5 (vs air)
Quality Level
vapor pressure
6 mmHg ( 20 °C)
product line
ReagentPlus®
assay
≥99%
form
liquid
autoignition temp.
662 °F
expl. lim.
11.4 %
dilution
(for general lab use)
refractive index
n20/D 1.452 (lit.)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Acetylacetone (2,4-pentanedione) is an organic compound containing two carbonyl groups and one active methylene group. It is mainly used as an intermediate in the synthesis of various chemical derivatives. It can also be used as a modifier for polyolefins, corrosion inhibitors, and labeling of radiotracers.
Application
Acetylacetone can be used as:
- A multifunctional ligand in the synthesis and feasible functionalization of gold nanoparticles (AuNPs).
- A reactant to synthesize 9,10-dihydroacridines by reacting with methyl acetoacetate and Morita-Baylis-Hillman acetates.
- A reagent in the synthesis of ZrO2(zirconium dioxide) via hydrolysis of Zr(OC3H7n)4. Acetylacetone controls the hydrolysis and condensation rates of alkoxides and thus, the nucleation and growth rates of oxides.
Packaging
Packaged in glass bottles
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
95.0 °F - closed cup
flash_point_c
35 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service