SAB4504651
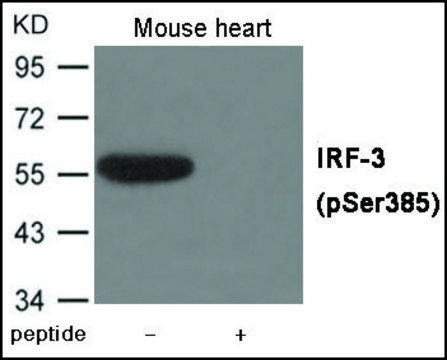
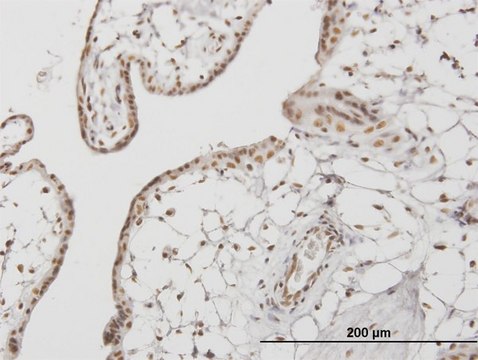
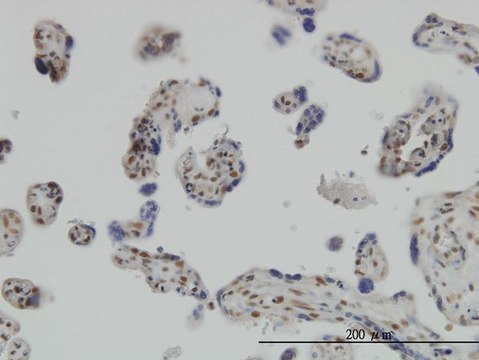
Anti-phospho-IRF-3 (pSer386) antibody produced in rabbit
affinity isolated antibody
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
mol wt
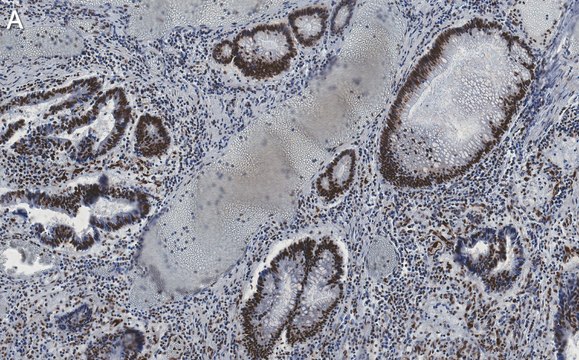
antigen 47 kDa
species reactivity
rat, human, mouse
concentration
~1 mg/mL
Related Categories
1 of 4
This Item | SAB1412471 | SAB1412666 | SAB1412667 |
|---|---|---|---|
| Gene Information human ... RUNX1(861) | Gene Information human ... RUNX1(861) | Gene Information human ... RUNX2(860) | Gene Information human ... RUNX2(860) |
| clone 3A8, monoclonal | clone 3A1, monoclonal | clone 1D2, monoclonal | clone 3F5, monoclonal |
| antibody form purified immunoglobulin | antibody form purified immunoglobulin | antibody form purified immunoglobulin | antibody form purified immunoglobulin |
| species reactivity human | species reactivity human | species reactivity human | species reactivity human |
| biological source mouse | biological source mouse | biological source mouse | biological source mouse |
| conjugate unconjugated | conjugate unconjugated | conjugate unconjugated | conjugate unconjugated |
Immunogen
Immunogen Range: 352-401
Features and Benefits
Physical form
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Yu program centers around the discovery of catalytic carbon–carbon and carbon–heteroatom bond forming reactions based on C–H activation. Target transformations are selected to enable 1) the use of simple and abundant starting materials such as aliphatic acids, amines and alcohols, and 2) disconnections that drastically shorten the synthesis of a drug molecule or a major class of biologically active compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service