581508-U
Ascentis® Si (5 µm) HPLC Columns
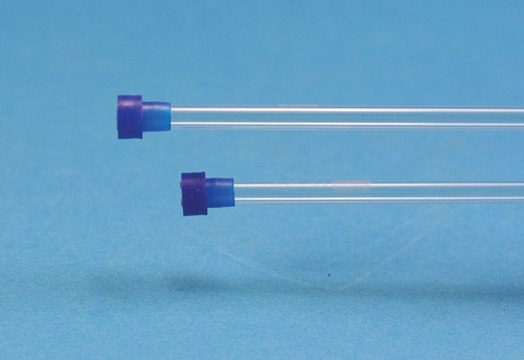
L × I.D. 10 cm × 2.1 mm, HPLC Column
About This Item
Recommended Products
Product Name
Ascentis® Si HPLC Column, 5 μm particle size, L × I.D. 10 cm × 2.1 mm
material
stainless steel column
Quality Level
agency
suitable for USP L3
product line
Ascentis®
feature
endcapped: no
manufacturer/tradename
Ascentis®
packaging
1 ea of
parameter
≤70 °C temp. range
400 bar pressure (5801 psi)
technique(s)
HPLC: suitable
LC/MS: suitable
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | 581514-U | 581513-U | 581502-U |
|---|---|---|---|
| matrix active group silica phase | matrix active group silica phase | matrix active group silica phase | matrix active group silica phase |
| separation technique hydrophilic interaction (HILIC), normal phase | separation technique hydrophilic interaction (HILIC), normal phase | separation technique hydrophilic interaction (HILIC), normal phase | separation technique hydrophilic interaction (HILIC), normal phase |
| particle size 5 μm | particle size 5 μm | particle size 5 μm | particle size 3 μm |
| L × I.D. 10 cm × 2.1 mm | L × I.D. 25 cm × 10 mm | L × I.D. 25 cm × 4.6 mm | L × I.D. 15 cm × 2.1 mm |
| pore size 100 Å | pore size 100 Å | pore size 100 Å | pore size 100 Å |
| agency suitable for USP L3 | agency suitable for USP L3 | agency suitable for USP L3 | agency suitable for USP L3 |
General description
The Ascentis Si is a high loading capacity silica with excellent peak shape. The Ascentis Si performs in both normal-phase and HILIC/ANP (aqueous normal phase) mode.
Legal Information
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service