C6624
Carbonic Anhydrase II human
recombinant, expressed in E. coli, buffered aqueous solution
Synonyme(s) :
CA-II, CA2, Carbonic Anhydrase 2
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro MDL:
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.54
Produits recommandés
Produit recombinant
expressed in E. coli
Niveau de qualité
Forme
buffered aqueous solution
Activité spécifique
≥5,000 units/mg protein
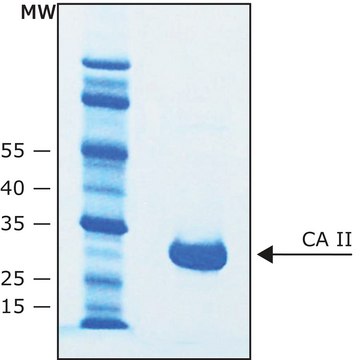
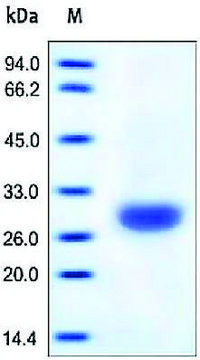
Poids mol.
large subunit 17-22 kDa
small subunit 10-12 kDa
Concentration
800-1000 μg/mL
Conditions d'expédition
wet ice
Température de stockage
−20°C
Informations sur le gène
human ... CA2(760)
Application
Human carbonic anhydrase II has been used in a study to assess quantitative imaging of mitochondrial and cytosolic free zinc levels in an in vitro model of ischemia/reperfusion. Human carbonic anhydrase II has also been used in a study to investigate a new scrubber concept for catalytic CO2 hydration.
Actions biochimiques/physiologiques
Carbonic anhydrase is a zinc metalloenzyme that catalyzes the hydration of carbon dioxide to carbonic acid. It is involved in vital physiological and pathological processes such as pH and CO2 homeostasis, transport of bicarbonate and CO2, biosynthetic reactions, bone resorption, calcification, and tumorigenicity. It is required for renal acidification. Its absence can lead to osteoporosis, renal tubular acidosis, and cerebral calcification. Therefore, this enzyme is an important target for inhibitors with clinical applications in glaucoma, epilepsy and Parkinson′s disease. In addition, it is being explored as a potential target for obesity and cancer. CAII has a molecular mass of approximately 30 kDa and is primarily present in type II pneumocytes. Due to this unique location it has been speculated that CAII is involved in pulmonary functions such as regulation of fluid secretion and facilitation of CO2 elimation. Sulfonamides, sulfamates and sulfamides are potent inhibitors of CA.
Définition de l'unité
One Wilbur-Anderson (W-A) unit will cause the pH of a 0.02 M Trizma buffer to drop from 8.3 to 6.3 per min at 0 °C. (One W-A unit is essentially equivalent to one Roughton-Booth unit.)
Forme physique
Supplied as a solution in 20 mM Tris, pH 7.5, with 150 mM NaCl.
Code de la classe de stockage
10 - Combustible liquids
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique