D7821
dPPA
≥98% (HPLC)
Synonym(s):
12-Deoxyphorbol 13-phenylacetate 20-acetate, DOPPA
About This Item
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
oil
color
colorless to light yellow
storage temp.
−20°C
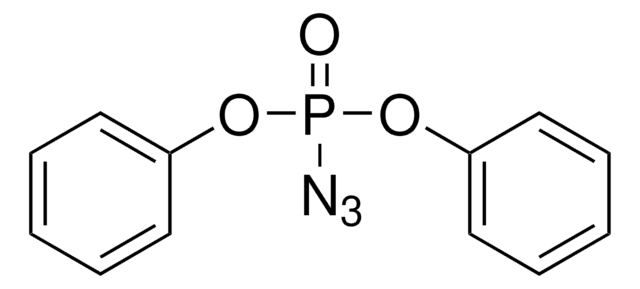
SMILES string
CC(C1=O)=C[C@]2([H])[C@]1(O)CC(COC(C)=O)=C[C@]3([H])[C@]2(O)[C@H](C)C[C@]4(OC(CC5=CC=CC=C5)=O)[C@H]3C4(C)C
InChI
1S/C30H36O7/c1-17-11-23-28(34,26(17)33)15-21(16-36-19(3)31)12-22-25-27(4,5)29(25,14-18(2)30(22,23)35)37-24(32)13-20-9-7-6-8-10-20/h6-12,18,22-23,25,34-35H,13-16H2,1-5H3/t18-,22+,23-,25-,28-,29+,30-/m1/s1
InChI key
MEDVHSNRBPAIPU-XMOZQXTISA-N
Application
- to treat transfected HT-22 cells to promote phosphorylation of p66Shc
- to treat HT-22 and B12 cells to study its effects on cell viability
- to treat neurons and measure primary cortical neurons using the 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
Biochem/physiol Actions
Features and Benefits
signalword
Danger
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 2 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Protein kinase C (PKC) is an AGC kinase that phosphorylates serine and threonine residues in many target proteins.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service